Posted on behalf of Sara Coles, Web-writer
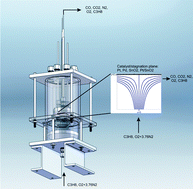 James Wiswall and his colleagues, working with Professor Margaret Wooldridge at the University of Michigan, USA, have been studying catalytic propane combustion. This reaction has important implications for areas such as power generation (for example, integration of catalysts directly into the internal combustion engine combustion chamber) and removal of volatile organic compounds (VOCs) from emissions.
James Wiswall and his colleagues, working with Professor Margaret Wooldridge at the University of Michigan, USA, have been studying catalytic propane combustion. This reaction has important implications for areas such as power generation (for example, integration of catalysts directly into the internal combustion engine combustion chamber) and removal of volatile organic compounds (VOCs) from emissions.
The group used a stagnation-point flow reactor to study the catalytic activity of platinum, palladium, tin dioxide and 90 wt% SnO2–10 wt% Pt for the combustion of propane under a variety of reaction conditions. Preliminary results suggest that the 90 wt% SnO2–10 wt% Pt catalyst provides significant activity and has similar trends in activity as a function of stagnation-plane temperature to those of Pt and Pd, whereas pure SnO2 shows no activity.
The researchers are optimistic that even better activity could be obtained if optimisation studies are carried out.
This development shows potential for Pt/SnO2 to be used as a fuel oxidation catalyst. For more detail, read the full article:
An experimental investigation of catalytic oxidation of propane using temperature controlled Pt, Pd, SnO2, and 90% SnO2–10% Pt catalysts
J. T. Wiswall, M. S. Wooldridge and H. G. Im
Catal. Sci. Technol., 2013, 3, 618–625










