Carbon dioxide is a problem. As a greenhouse gas it contributes towards global warming, and its ever-growing concentrations in the atmosphere are cited as a cause of anthropomorphic climate change. But now a team of researchers from the University of Bath have opened up the idea of using carbon dioxide as a useful potential feedstock; a useful chemical resource rather than a troublesome waste product.
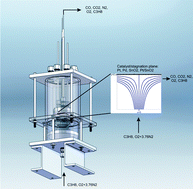
Davide Mattia, who leads the research team, has taken inspiration from the well-known Fischer-Tropsh process, which uses an iron catalyst to react hydrogen with carbon monoxide, producing a mixture of alkanes that can be used as fuel. Mattia’s catalyst, which works with both carbon monoxide and carbon dioxide, is also iron based, taking the form of iron nanoparticles embedded on carbon nanotubes. It has a rather unique method of preparation, which is not only simpler to carry out, but results in a more effective catalyst.

To read the full story, story please visit Chemistry World.
High CO2 and CO conversion to hydrocarbons using bridged Fe nanoparticles on carbon nanotubes
Justin P. O’Byrne, Rhodri E. Owen, Daniel R. Minett, Sofia I. Pascu, Pawel K. Plucinski, Matthew D. Jones and Davide Mattia
Catal. Sci. Technol., 2013, Advance Article
DOI: 10.1039/C3CY20854K