This month sees the following articles in Catalysis Science & Technology that are in the top ten most accessed:-
Catalytic activity of unsupported gold nanoparticles
Yusuke Mikami , Amarajothi Dhakshinamoorthy , Mercedes Alvaro and Hermenegildo García
Catal. Sci. Technol., 2012, Advance Article DOI: 10.1039/C2CY20068F
Graphene-based materials for catalysis
Bruno F. Machado and Philippe Serp
Catal. Sci. Technol., 2012,2, 54-75 DOI: 10.1039/C1CY00361E
Advances in conversion of hemicellulosic biomass to furfural and upgrading to biofuels
Saikat Dutta , Sudipta De , Basudeb Saha and Md. Imteyaz Alam
Catal. Sci. Technol., 2012,2, 2025-2036 DOI: 10.1039/C2CY20235B
Role of mixed metal oxides in catalysis science—versatile applications in organic synthesis
Manoj B. Gawande , Rajesh K. Pandey and Radha V. Jayaram
Catal. Sci. Technol., 2012,2, 1113-1125 DOI: 10.1039/C2CY00490A
Conversion of lignocellulose into renewable chemicals by heterogeneous catalysis
Hirokazu Kobayashi , Hidetoshi Ohta and Atsushi Fukuoka
Catal. Sci. Technol., 2012,2, 869-883 DOI: 10.1039/C2CY00500J
Enhanced photocatalytic degradation of methylene blue by ZnO–reduced graphene oxide–carbon nanotube composites synthesized via microwave-assisted reaction
Tian Lv , Likun Pan , Xinjuan Liu and Zhuo Sun
Catal. Sci. Technol., 2012, Advance Article DOI: 10.1039/C2CY20023F
Direct coupling of alcohols to form esters and amides with evolution of H2 using in situ formed ruthenium catalysts
Martin H. G. Prechtl , Kathrin Wobser , Nils Theyssen , Yehoshoa Ben-David , David Milstein and Walter Leitner
Catal. Sci. Technol., 2012,2, 2039-2042 DOI: 10.1039/C2CY20429K
Self-assembled monolayer coated gold-nanoparticle catalyzed aerobic oxidation of a-hydroxy ketones in water: an efficient one-pot synthesis of quinoxaline derivatives
Tamalika Bhattacharya , Tridib K. Sarma and Sampak Samanta
Catal. Sci. Technol., 2012, Advance Article DOI: 10.1039/C2CY20438J
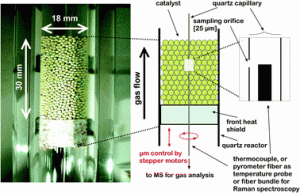
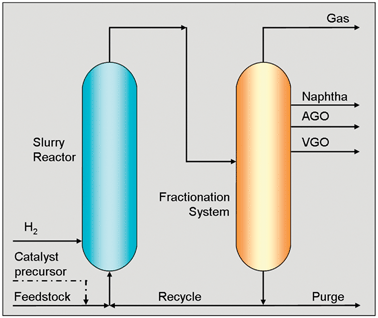
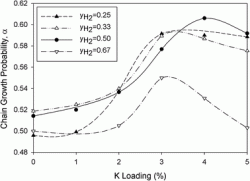
Diesel fuel from biomass
Carlo Perego and Marco Ricci
Catal. Sci. Technol., 2012,2, 1776-1786 DOI: 10.1039/C2CY20326J
Challenges in the catalytic synthesis of cyclic and polymeric carbonates from epoxides and CO2
Paolo P. Pescarmona and Masoumeh Taherimehr
Catal. Sci. Technol., 2012, Advance Article DOI: 10.1039/C2CY20365K
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Catalysis Science & Technology ? Then why not submit to us today or alternatively email us your suggestions.
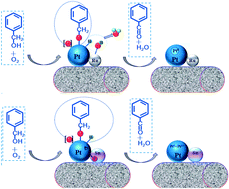
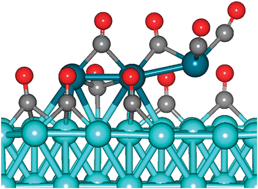 A collaboration of scientists in China, Germany and Singapore have used a computational model to study palladium leaching processes under a carbon monoxide atmosphere.
A collaboration of scientists in China, Germany and Singapore have used a computational model to study palladium leaching processes under a carbon monoxide atmosphere.