Fuel conversion from biomass to liquid hydrocarbons is a fast moving area of research and presents an opportunity to decrease our dependence on fossil fuels and move towards a more carbon neutral fuel economy. For use in transportation there are currently a range of strategies being considered to create liquid fuel from different biomass feedstocks (see Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels).
John C. Gordon, L. A. ‘‘Pete’’ Silks and colleagues have recently investigated a method of opening biomass-derived furan rings, under mild conditions, using homogeneous Bronsted acid catalysis.
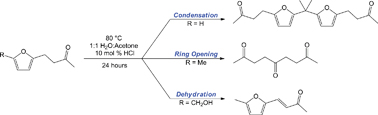
The products observed during acid catalyzed ring opening of furan containing biomass-derived substrates are strongly influenced by furan substituents.
When generating fuel from non-food biomass there are many chemical hurdles to overcome, including the breakdown of lignocellulose and subsequent deoxygenation and hydrogenation of the resulting products. Gasification followed by Fischer–Tropsch reaction is a promising route to biomass conversion, but requires high temperatures and initial oxidation of the biomass.
An important challenge is the opening of ring structures.
While cellulose based biofuel precursors can be hydrolyzed under mild conditions, subsequent dehydration of these sugars leads to the generation of furans and aldehydes. In their Hot Article John C. Gordon et al. have investigated experimentally and theoretically the ring opening mechanism of furans on molecules derived from biomass, using acid catalysis <100oC. This important study gives insight into the ring opening process which is necessary to create linear alkane chains for use as liquid fuels.
Download their article for free to find out more
Functional group dependence of the acid catalyzed ring opening of biomass derived furan rings: an experimental and theoretical study
Christopher R. Waidmann, Aaron W. Pierpont, Enrique R. Batista, John C. Gordon, Richard L. Martin, L. A. “Pete” Silks, Ryan M. West and Ruilian Wu
DOI: 10.1039/C2CY20395B











