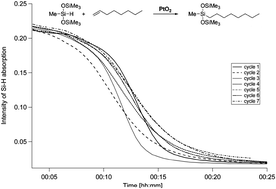
Compared to the usual homogeneous catalysts PtO2 dissolves only to a small degree after reaction with silane. The un-reacted solid catalyst is then easily separated from the reaction mixture by simple decantation or filtration and so can be used for subsequent runs. The observation of an induction period in every cycle indicates that the active species is formed in situ before the reaction can take place and new material has to dissolve for each run.
The active species is formed by reduction of PtO2 with the silane and is soluble in the reaction mixture. The solubility behavior together with the high activity allows a ‘‘self-dosing’’ of the catalyst—leading to little waste of precious metal in contrast to other ‘‘homogeneous’’ (i.e. better soluble) Pt-based catalysts making it potentially useful for industrial applications.
Read more for FREE throughout 2012 at:
PtO2 as a “self-dosing” hydrosilylation catalyst
Sophie Putzien, Eckhart Louis, Oskar Nuyken and Fritz E. Kühn
Catal. Sci. Technol., 2012, Advance Article
DOI: 10.1039/C2CY00367H










