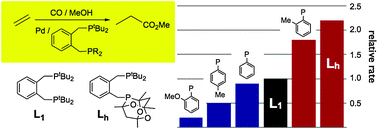
Methyl propanoate of course. The conversion of ethene to methyl propanoate (MeP) is commercially catalysed using diphosphine Pd catalysts with carbon monoxide and methanol. Bulky R groups on the diphosphine ligand can create highly selective, efficient organometallic catalysts, tertiary butyl and aryl groups tend to provide the necessary steric bulkiness and make for good ligand substituents. Paul G. Pringle et al. report in their HOT Article the use of hetero diphosphine ligands – their investigation has not only led to the discovery of complexes which improve upon the industrially used catalysts but also gives some insight into possible structure-activity relationships. The team’s discoveries open up new opportunities for ligand design and the rational synthesis of improved organometallic catalysts for ethene methoxycarbonylation, remarkably the group found that only one bulky phosphine donor is necessary for effecient catalysis, for more details read the full article below.
Efficient and chemoselective ethene hydromethoxycarbonylation catalysts based on Pd-complexes of heterodiphosphines o-C6H4(CH2PtBu2)(CH2PR2)
Tamara Fanjul, Graham Eastham, Mairi F. Haddow, Alex Hamilton, Paul G. Pringle, A. Guy Orpen, Tom P. W. Turner and Mark Waugh
Catal. Sci. Technol., 2012, Advance Article
DOI: 10.1039/C1CY00409C