The beginning of the new century, in chemistry, has been often linked to the renaissance of organocatalysis as enthusiastically reported by articles appearing on Angewandte Chemie and Chemical Society Reviews in the past few years. Although presenting several advantages over traditional metal-based catalysts, such as high stereoselectivity, ease of synthesis and handling, and the general feeling of being more environmentally benign, they yet have to persuade industry into their large scale application.
Some of the reasons behind the fall-out of industry for organocatalysts lie in the lower efficiency they exhibit, the major difficulties of catalyst separation, recovery and recycling. Attempts to covalently bind organocatalysts on polymeric and other supports addressed the problems of separation but often resulted in a loss of activity triggered by modifications of structure of the catalyst.
 In an interesting perspective appeared on Cat. Sci. Technol., Luo, Zhang and Chen presented an overview of alternative immobilisation techniques based on non-covalent bonds.
In an interesting perspective appeared on Cat. Sci. Technol., Luo, Zhang and Chen presented an overview of alternative immobilisation techniques based on non-covalent bonds.
As stated by the authors, the loss of activity on covalently bound organocatalysts is likely to depend on the changes in the structure and chemical properties that these small organic molecules undergo when bound to the support. Non-covalent immobilisation, on the other hand, seems to circumvent this problem and, despite other shortcomings, might be a promising field of study.
Examples of acid-base immobilisation presented include the use of solid acid like polyoxometalates or polystyrene sulfonic acids as the support, used with chiral amines-based catalysts in the aldol reaction and Michael additions, where recovery of the catalyst could be achieved by precipitation with diethyl ether. Other immobilisation strategies presented are the incorporation of the organocatalyst into a phase transfer catalysts (PTC) and the immobilisation into clays. The latter is achieved on materials such as montmorillonite, which comprises negatively charged layers alternated with layers of Na+ species.Using a cation exchange process, molecules such as proline and proline derived structures could be immobilized in the interlayers and the resulting material successfully used in aldol reactions. Another family of supports is represented by ionic liquids, where an organocatalysts is used as the anionic partner in the structure, creating chiral ionic liquid s that had been used to catalyse Mannich-type and aza-Diels-Alder reactions. Other supports mentioned are self-supported gel-type organocatalysts, biphasic immobilisation and techniques based on hydrophobic interactions, using cyclodextrines as the supports.
Several detailed examples and references for each category, inviting the interested reader to further reading.
In the authors` words – the non-covalent immobilisation is anticipated to provide a viable solution to enhance the applications of organocatalysis with “practical” credentials.
Read the full article here
Perspective
Non-covalent immobilisation of asymmetric organocatalysts
Long Zhang, Sanzhong Luo and Jin-Pei Cheng
Catal. Sci. Technol., 2011, Advance Article
DOI: 10.1039/C1CY00029B












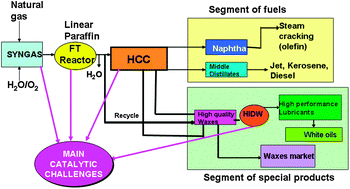 This Perspective article by Eduardo Falabella Sousa-Aguiar, from the Federal University of Rio de Janeiro, and colleagues, has been chosen as a Catalysis Science & Technology Hot article.
This Perspective article by Eduardo Falabella Sousa-Aguiar, from the Federal University of Rio de Janeiro, and colleagues, has been chosen as a Catalysis Science & Technology Hot article.


_410_tcm18-202980.jpg)
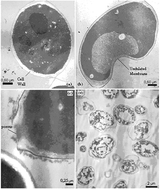 In this recently published Catalysis Science & Technology article, Tatiana Felix Ferreira and colleagues from the Federal University of Rio de Janeiro extract beta-glucan from yeast cell walls.
In this recently published Catalysis Science & Technology article, Tatiana Felix Ferreira and colleagues from the Federal University of Rio de Janeiro extract beta-glucan from yeast cell walls.
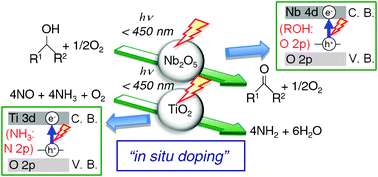 This Catalysis Science & Technology Hot article, reviews how photo-assisted selective NO reduction with ammonia over titania and the photooxidation of alcohols over Nb2O5 works.
This Catalysis Science & Technology Hot article, reviews how photo-assisted selective NO reduction with ammonia over titania and the photooxidation of alcohols over Nb2O5 works.