 Enzymes are by far the most active and selective catalysts known to chemistry but although progress has been made to incorporate them into industrial use (i.e. in batch reactors, fixed bed immobilised enzyme reactors), their intrinsic biological nature makes them too substrate-specific and limits them to a reduced range of operational conditions.
Enzymes are by far the most active and selective catalysts known to chemistry but although progress has been made to incorporate them into industrial use (i.e. in batch reactors, fixed bed immobilised enzyme reactors), their intrinsic biological nature makes them too substrate-specific and limits them to a reduced range of operational conditions.
In an attempt to bypass these limitations, scientists developed bio-inspired catalysts: structures designed upon the active sites of enzymes but lacking the organic framework that characterise them, making them easier to heterogenise, recover and more resilient to harsh conditions than their natural counterpart.
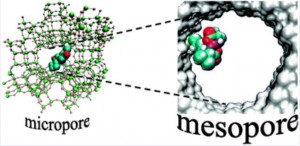
To reintroduce the selectivity given by the enzyme`s structure, these catalysts can be incorporated into micro- or mesoporous supports such as silica and zeolites exploiting ionic interactions between metal and support or different anchoring techniques.The selectivity is mostly size-driven, dictated by the dimension of the pores in the material, or induced by the presence of chiral ligands on the catalyst.
In addition to metal complexes, natural and modified aminoacids can also be chemically bound to porous supports, resulting in efficient catalysis of a wide range of reactions. The characterisation of the active sites, though, is made more difficult by the lack of appropriate techniques that clearly discriminate the catalytic species from the support.
In-depth coverage of the topic and future perspectives can be found here.
Design strategies for engineering selectivity in bio-inspired heterogeneous catalysts
David J. Xuereb and Robert Raja
Catal. Sci. Technol., 2011, Advance Article
DOI: 10.1039/C0CY00088D, Perspective










