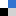Scientists from India used visible light activation of persulphate by a ruthenium(II) complex to investigate it’s ability to cause degradation of organic contaminants.
The authors note that to take this appraoch forward and make it more practical for actual application in the environment, strategies for removal of the ruthenium photosensitizer and its degradation products need to be developed.
Read the full article for free until the 3rd January 2013!
Photodegradation of methyl orange and photoinactivation of bacteria by visible light activation of persulphate using a tris(2,2′-bipyridyl)ruthenium(II) complex, Gokulakrishnan Subramanian, Priyadarshini Parakh and Halan Prakash, Photochem. Photobiol. Sci., 2013, DOI: 10.1039/C2PP25316J