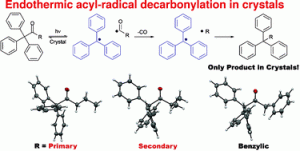
 In this recently published communication John R. Scheffer and Miguel A. Garcia-Garibay and their teams from the University of British Columbia and the University of California Los Angeles demonstrate that the solid state photoexcitation of several triphenylmethylalkyl ketones results in the loss of CO and the exclusive formation of radical–radical combination products. Furthermore, differences in reactivity suggest a stepwise mechanism with the unprecedented formation of primary and secondary radicals in some of the radical pair intermediates in the solid state.
In this recently published communication John R. Scheffer and Miguel A. Garcia-Garibay and their teams from the University of British Columbia and the University of California Los Angeles demonstrate that the solid state photoexcitation of several triphenylmethylalkyl ketones results in the loss of CO and the exclusive formation of radical–radical combination products. Furthermore, differences in reactivity suggest a stepwise mechanism with the unprecedented formation of primary and secondary radicals in some of the radical pair intermediates in the solid state.
Interested in knowing more? Read the full article here. Free until October 21st!
Stable radicals during photodecarbonylations of trityl-alkyl ketones enable solid state reactions through primary and secondary radical centers
Gregory Kuzmanich, Arunkumar Natarajan, Yanhui Shi, Brian O. Patrick, John R. Scheffer and Miguel A. Garcia-Garibay
Photochem. Photobiol. Sci., 2011, DOI: 10.1039/C1PP05240C