Hollow micro-/nanostructures have a wide range of potential applications, including catalysis, drug delivery, sensors and fuel cells. This is thanks to their unique array of properties such as high specific surface area, low density and high loading capacity.
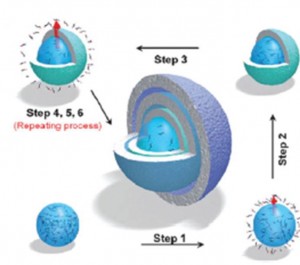
As any child will tell you, two sweets are better than one, while three or four are better still, and the same holds true for the number of shells in a multi-shelled hollow micro-/nanostructure. These multi-shelled structures should have significantly prolonged release times for drug delivery and improved performance in heterogeneous catalysis, lithium ion batteries and photocatalysis applications. However, with increased complexity come increased synthetic challenges.
In their recent review, titled, ‘Multi-shelled hollow micro-/nanostructures’, Dan Wang et. al. describe the myriad of synthetic approaches for multi-shelled hollow micro-/nanostructures, before focusing on their compositional and geometric manipulation, as well as the range of potential applications. Finally, the authors look at the future challenges in the area, which include: expanding the components that can be used to make multi-shells, multi-shells whose individual shells are different, and control of inter-shell spacing.
Although other recent review articles have discussed hollow micro-/nanostructured materials, this is the first to focus exclusively on multi-shelled hollow structures.
If you are interested in working on any of these challenges or others that the authors highlight, this review is a perfect starting place to get up to speed.
To read the details, check out the Chem Soc Rev article in full:
Multi-shelled hollow micro-/nanostructures
Jian Qi, Xiaoyong Lai, Jiangyan Wang, Hongjie Tang, Hao Ren, Yu Yang, Quan Jin, Lijuan Zhang, Ranbo Yu, Guanghui Ma, Zhiguo Su, Huijun Zhao and Dan Wang
Chem. Soc. Rev., 2015, 44, DOI: 10.1039/C5CS00344J