Luis G. Rigat-Brugarolas1,2, Antoni Homs-Corbera1,2 and Josep Samitier1,2,3
1 Nanobioengineering group, Institute for Bioengineering of Catalonia (IBEC), Barcelona, Spain
2 Centro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN), Zaragoza, Spain.
3 Department of Electronics, Barcelona University (UB), Martí I Franques, 1, Barcelona, 08028, Spain.
Why is this useful?
Nowadays it is common to fabricate multi-layered microfluidic microdevices by means of photolithographic techniques to create sophisticated structures allowing novel functionalities.1,2 Without any doubt, one of the critical steps in this manufacturing process is the alignment of the different transparent layers to perform the final device.
Several approaches have been made and studied to obtain a correct structuring of the three-dimensional device like, for example, the use of gold deposition, by means of sputtering techniques, for drawing the alignment marks in the substrate,3 or using expensive mask aligners with integrated alignment protocols. Those methods are expensive and laborious. In this work we present a novel, simple and non-time-consuming methodology for drawing alignment marks using Ordyl SY330 negative photofilm, a photoresist that can be easily displayed in the substrate and, thanks to its green color, it can be readily seen in a standard microscope.
What do I need?
- Common items and devices used in photolithographic processes (mask aligner, hot plates and transparent substrate)
- Sheets of Ordyl SY330 negative photofilm.
- Ordyl Developer, SU-8 Developer or acetone.
- Photomask with the alignment marks details.
- Hot laminator.
What do I do?
A scheme of the alignment marks’ fabrication process can be seen in Figure 1. The steps are as follows:
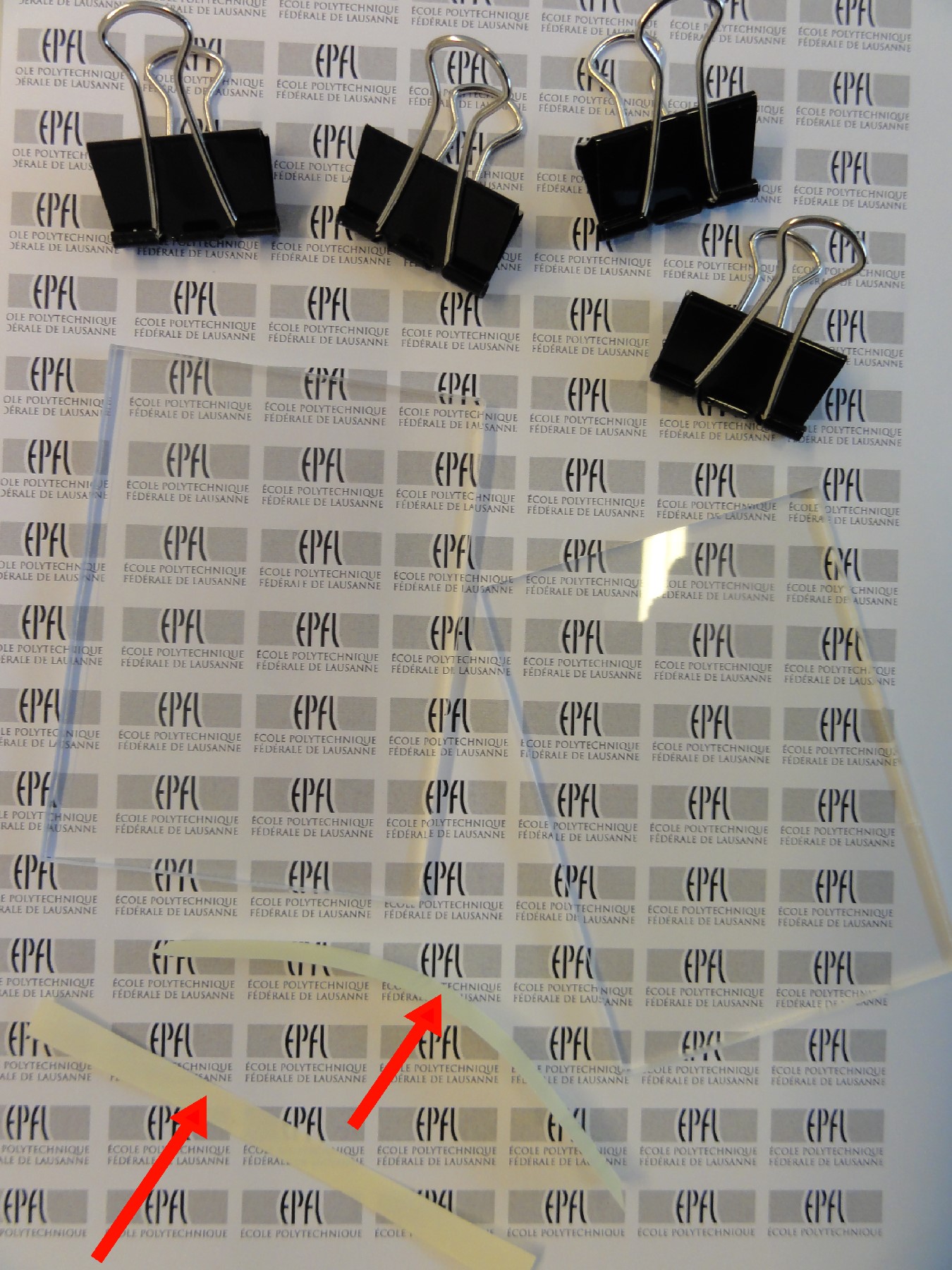
- Dispose the Ordyl photofim over the substrate (Figures 1A-B and 2).
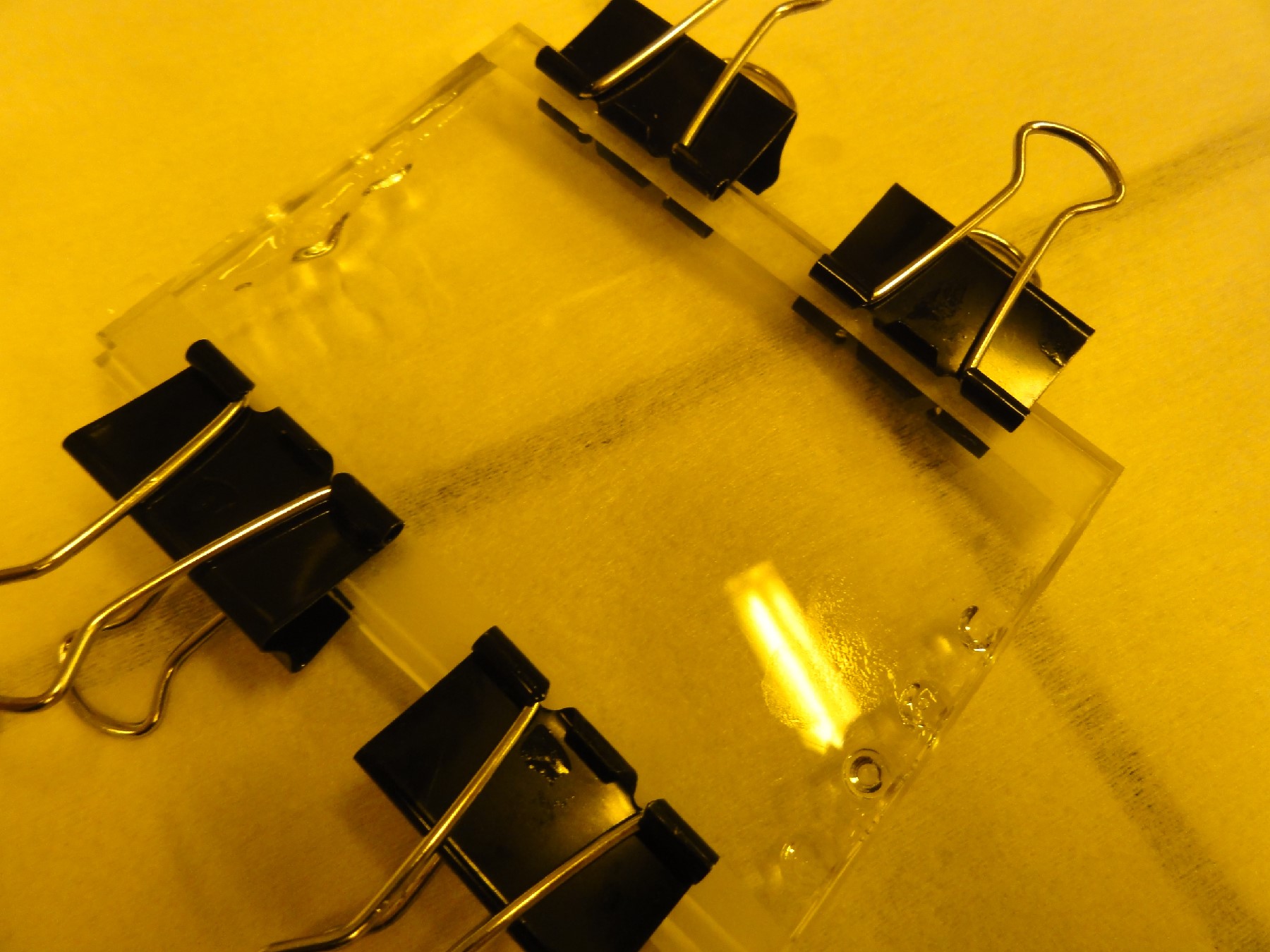
- Introduce both the substrate and the Ordyl film in a hot laminator in order to attach it firmly (see Figure 3).
- For the exposure, use an acetate or chrome-on-glass photomask (an example can be seen in Figure 4) with the design of the alignment marks. Because Ordyl is a negative photoresist, the design should have the marks in transparent in order to polymerize them and draw them in the substrate (Figure 1C).
- After exposure to UV Light (Figures 1D-E), an Ordyl Developer is needed (or, failing this, SU-8 developer or acetone), for having the final marks drawn in the substrate (an example can be seen in Figure 5).

Fig 1: Scheme of the fabrication process of the Ordyl alignment marks.

Fig 2: Placement of Ordyl photofilm on a microscope slide.

Fig 3: The hot laminator is used to attach the Ordyl film to the substrate.

Fig 4: Photomask with the design of the alignment marks.

Fig 5: Example of Ordyl alignment marks on a glass substrate.
Reference
[1] Dongeun Huh, Hyun Jung Kim, Jacob P Fraser, Daniel E Shea, Mohammed Khan, Anthony Bahinski, Geraldine A Hamilton and Donald E Ingber. Microfabrication of human organs-on-chips. Nature protocols. 2013 Nov. 11, vol.8. Doi:10.1038/nprot.2013.137.
[2] Michael P Cuchiara, Alicia CB Allen, Theodore M Chen, Jordan S Miller, Jennifer L West. Multilayer microfluidic PEGDA hydrogels. Biomaterials. 2010 31 5491e5497
[3] Eugene JH Wee, Sakandar Rauf, Kevin MS Koo, Muhammad JA Shiddiky, and Matt Trau. µ-eLCR: A Microfabricated Device for Electrochemical Detection of DNA Base Changes in Breast Cancer Cell Lines. LabChip. 2013 Nov 21;13(22):4385-91. DOI: 10.1039/c3lc50528f.