Sahl Sadeghi1* and Meltem Elitas1
1 Faculty of Engineering and Natural Sciences, Sabanci University, 34956, Istanbul, Turkey
* Sahl Sadeghi wrote the paper.
Purpose
Lab-on-a-chip (LOC) devices significantly contribute different disciplines of science. Polydimethylsiloxane (PDMS) is one of the main materials, which is widely used for the fabrication of biological LOCs, due to its biocompatibility and ease of use. However, PDMS and some other polymeric materials are intrinsically water repellant (or hydrophobic), which results in difficulties in loading and operating LOCs. The eminent consequence of hydrophobicity in LOCs for biological systems is the entrapment of air bubbles in microfluidic channels. Although the oxygen plasma treatment of PDMS reduces the surface hydrophobicity for a certain period of time, the hydrophilic property of PDMS vanishes over time1. The persistent problem of bubbles in the microfluidics led to several studies conducted to overcome it. Some of these solutions suggested implementing bubble traps2,3, surface treatment of LOCs through hydrophilic coatings4, and using actively controlled bubble removal systems 5,6.
Although the aforementioned design complexities are introduced to LOCs in order to reduce the clogging problem caused by the bubbles, these modifications also result in higher production cost, complex operation, and long device preparation time. In many single-cell experiments without losing or damaging the rare cells, these cells needs to be introduce into the LOCs. Here, we present a simple method that enables loading a small number of cells without introducing bubbles in the microfluidics channels.
Materials
| · PDMS | (Dow Corning Sylgard 184 Silicon Elastomer Kit) |
| · Pipette tips | (20-200 ul, Eppendorf, # 3120000917) |
| · Pipetman | (Gilson, P200, #69989-5) |
| · Aqueous ethanol 70% | (ZAG Chemistry) |
| · Cell culture medium | (DMEM, PAN Biotech, #P04-01548) |
| · Mammalian cells | (MCF7, ATCC-HTB-22) |
| · Sterile syringe | (BD 10 ml Syringe, Luer-Lok Tip, #300912) |
| · Sterile Hamilton syringe | (Hamilton, 100 ul SYR, #84884) |
Procedure
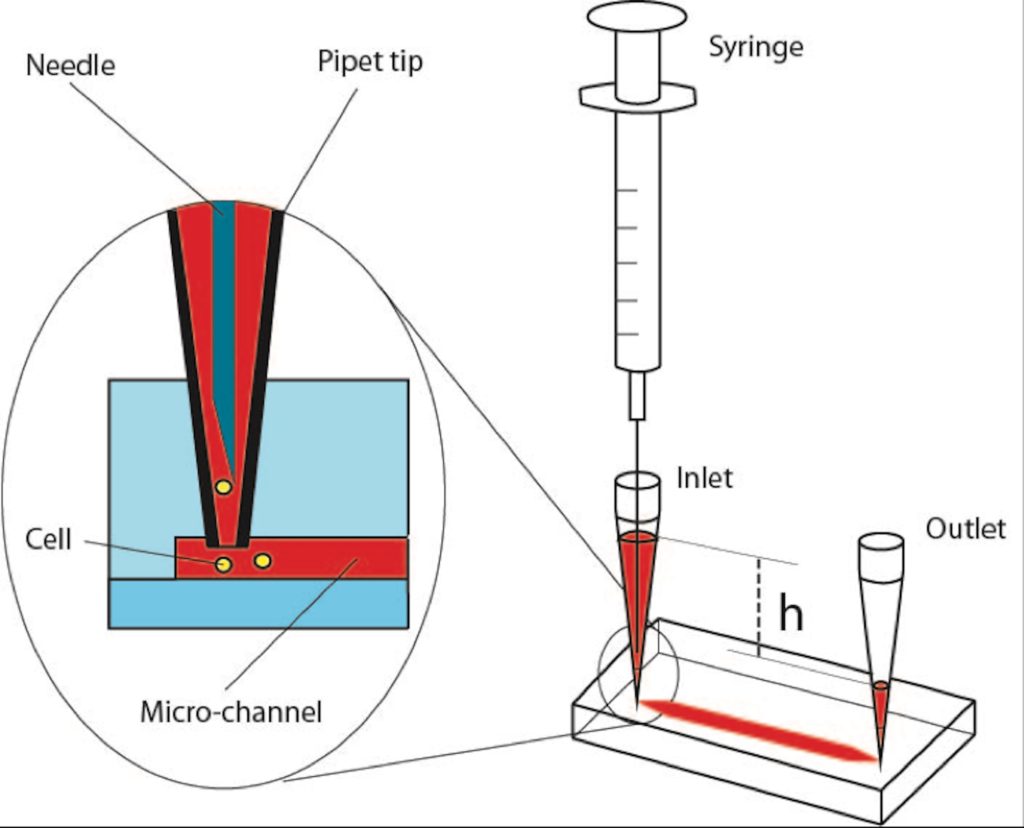
Step 1: Insert two 200-ul pipet tips at the inlet and outlet ports of the PDMS device as illustrated in Figure 1.
Step 2: Introduce a 70% aqueous ethanol into the inlet-pipet tip using a pipetman. Thus, the inner surface of microfluidic channels will be disinfected and the fluid flow will be tested within the micro channels as it is applied in many other protocols for LOCs7,8.
Step 3: Gently apply pressure pressing the pipetman to force ethanol solution flow through the micro channels and cavities of the PDMS device. Take care to avoid applying negative pressure from the outlet-pipet tip, which might create air leakage through the pipet connections. Besides, applying a negative pressure will directly affect the amount of a gas dissolved in the liquid according to Henry’s law9 that might contribute formation of more bubbles inside the micro-channels. The positive pressure will facilitate removal of the air bubbles via dissolving them.
Step 4: After flushing the chip with ethanol solution, inspect the chip to ensure bubble removal. In case of air bubbles, repeat the steps 2 and 3.
Step 5: Fill the syringe (10 ml) with medium or phosphate buffered saline (PBS). Take care to remove the air bubbles inside the syringe, mount and lock the needle on the syringe. Then, flow medium through the needle too make sure the needle is full of medium without any bubble. Insert the needle in the inlet-pipet tip; gently apply positive pressure to replace the ethanol with medium. Next, collect the excess medium from the outlet-pipet tip.
Step 6: Fill the inlet pipet with fresh medium in such a way that due to certain height (h) between the levels of the medium in the inlet and outlet pipet tips, very slow medium flow will be established inside the micro-channels.
Step 7: Load your cells into the Hamilton syringe and take care to ensure that there is no air bubble inside its needle and syringe. Insert the needle of the Hamilton syringe into the inlet-pipet tip as explained in Step 5, Figure 1. Introduce the cells via applying gentle positive pressure to the syringe. Established flow streams in the PDMS chip will deliver the released cells to the desired positions in the chip. Flow rate can be arranged adjusting the applied positive pressure and amount of medium collected in the inlet and outlet pipet tips. The excess supernatant from the outlet-pipet tip can be collected, and fresh medium can be supplied through the inlet-pipet tip during the experiment.
References
- Tan, S.H., N.T. Nguyen, Y.C. Chua, and T.G. Kang,. Biomicrofluidics, 2010. 4(3).
- Zheng, W.F., Z. Wang, W. Zhang, and X.Y. Jiang,. Lab on a Chip, 2010. 10(21): p. 2906-2910.
- Wang, Y., D. Lee, L.S. Zhang, H. Jeon, J.E. Mendoza-Elias, T.A. Harvat, S.Z. Hassan, A. Zhou, D.T. Eddington, and J. Oberholzer, . Biomedical Microdevices, 2012. 14(2): p. 419-426.
- Wang, Y.L., C.E. Sims, and N.L. Allbritton,. Lab on a Chip, 2012. 12(17): p. 3036-3039.
- Karlsson, J.M., M. Gazin, S. Laakso, T. Haraldsson, S. Malhotra-Kumar, M. Maki, H. Goossens, and W. van der Wijngaart,. Lab on a Chip, 2013. 13(22): p. 4366-4373.
- Cortes, D.F., T.-X. Tang, D.G.S. Capelluto, and I.M. Lazar,. Sensors and Actuators B: Chemical, 2017. 243: p. 650-657.
- Benavente-Babace, A., D. Gallego-Perez, D.J. Hansford, S. Arana, E. Perez-Lorenzo, and M. Mujika,. Biosensors & Bioelectronics, 2014. 61: p. 298-305.
- Yesilkoy, F., R. Ueno, B.X.E. Desbiolles, M. Grisi, Y. Sakai, B.J. Kim, and J. Brugger,. Biomicrofluidics, 2016. 10(1).
- Henry, W., Phil. Trans. R. Soc. Lond., 1803. 93: 29–274.