Sopan M. Phapal, Tuhina Vijay and P. Sunthar
Chemical Engineering Department, Indian Institute of Technology Bombay, Powai-400 076, India.
Why is this useful?
There are several ways to connect macro to micro interfaces (e.g., from syringe pump to microfluidic chip) in the miniaturization field. The ideal connector should withstand very high pressure and not leak. Commercially available Nanoports are often used as connectors but they are expensive, sometimes become clogged when glue is used, and cannot be reused. In this tip we present an easy and inexpensive way to produce a macro to micro interface connector which can withstand high pressure and is glue free.
What do I need?
• 2.5 ml plastic BD syringe with Luer-Lok (Luer tip ID at back 2.33 mm/0.095”; front ID 1.75 mm/0.07”)
• 16G SS needles (OD= 1.65 mm/0.065”)
• Grinder with hard abrasive disk to cut/ blunt the needles
• 16G PTFE tubing, Hamilton, Cat No.20916 ; OD=1.9 mm/0.075”; ID=1.2 mm/0.047”[1]
• Surgical knife/cutter to cut the Luer part of the syringe
• Glue (Araldite).
A syringe contains a male Luer part and the needle head is its counter female Luer part. There are two types of Luer fittings, one Luer-slip which when pressed together, fits in each other by friction; and the other is Luer-Lok which has threads in the male part and the female part (needle head) screws in to it (Figure 1). We used both Luer-slip and Luer-Lok syringe tips, but the Luer-Lok is better for high pressure application.
What do I do?
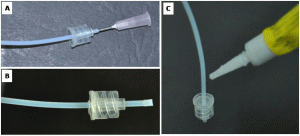
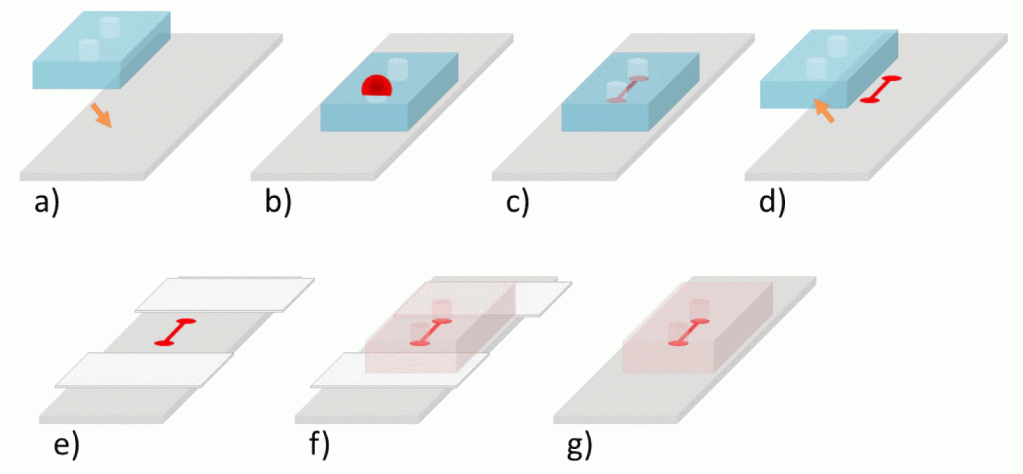
1. Make the needles blunt with the help of grinder (Fig. 2A & B). Cut the Luer-Lok tip of the plastic syringe with a sharp blade/cutter (Fig. 2C).
2. Prepare the male Luer part. Insert the PTFE tube (OD= 1.9mm) into Luer-Lok tip (back ID= 2.3 mm and front ID is 1.75 mm) as shown in Fig. 3A. Now heat the 16G SS blunt needle and insert it into the PTFE tube up to some mm and remove it (Fig. 3B). Because of this, the tip of the tube becomes wider than Luer-Lok ID, fitting tight mechanically. Pull the Luer-Lok up to the wide tip of PTFE tube and place a drop of araldite at the back side of it (Fig. 3C). This will cause a tight fit of the Luer-Lok tip with the PTFE tube.
3. Preparation of the female Luer part. Heat the 16G SS blunt needle (OD= 1.65 mm) and insert it into 16G PTFE tube (ID=1.2mm), as shown in Fig. 4. As the needle OD is bigger than tube ID, it will permanently fit into the PTFE tube upon cooling.
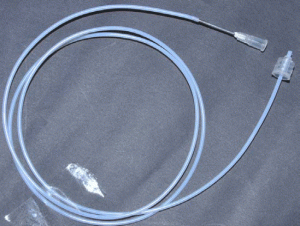
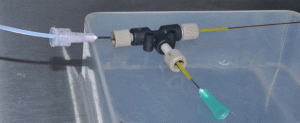
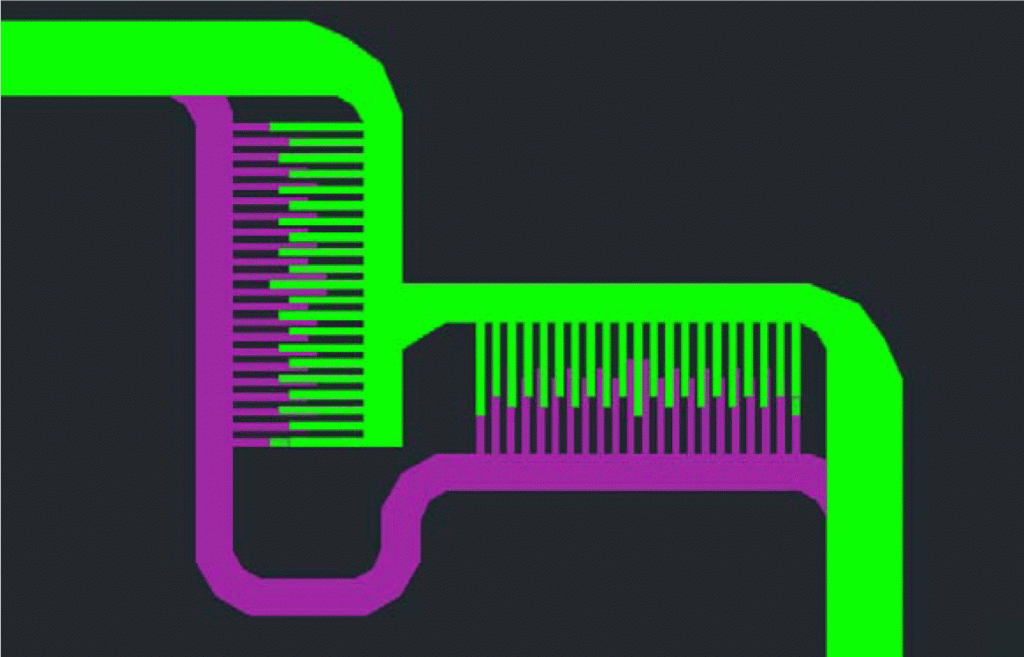
4. Examples of connecting the male Luer part of tubing into a needle head (female Luer part) which is acting as an inlet of micro channel assembly (Fig. 5). Example of the use of macro to micro interface tubing to connect a syringe to a microchip: screw fit the needle head (female Luer) into the male Luer part on the syringe placed on syringe pump and the male Luer-Lok part will screw fit into the inlet needle (counter fitting female part) of microfluidic chip (Fig. 6).
References
[1]. http://www.hamiltoncompany.com
Acknowledgements
We would like to acknowledge a grant provided by the Department of Science and Technology, Government of India (07-DS-032).