Amongst the myriad uses of metal-organic frameworks (MOFs) and coordination polymers, separation of complex mixtures, either gaseous or in solution, is one of the most promising applications due to the regular array of well-defined pores. Integration of specific points of interaction that are complementary to the targeted guest species can provide highly selective materials. In particular, precise control of the 3D space within pores may provide a mechanism for enantioselective discrimination for which the exact spatial arrangement of multiple sites of interaction is paramount.
The vast majority of research in this area is driven by 3D metal-organic frameworks, often those possessing permanent porosity. However, for solution-based applications this need not be a prerequisite and materials comprising lower dimensionality coordination polymers may be just as effective is they contain solvent-filled pores.
Recently the group of David Turner at Monash University has shown that a 1D coordination polymer, with pores formed by alignment of loops within the 1D chain, is capable of sorbing 1-phenylethanol with a good degree of enantioselectivity (Figure 1). A closely related material shows no such selectivity, and a reduced uptake capacity, highlighting the importance of structural match between the host framework and the analyte. Ground samples showed higher uptake than unground crystals for the active material, suggesting an influence of surface area or ease of permeation into the solid. Both static (soaking) and dynamic (“micro-LC”) methods showed enhanced uptake of one enantiomer from a racemic solution of 1-phenylethanol, albeit not perfect separation. The group was also able to crystallographically determine the binding site of the preferred enantiomer (Figure 2), showing an array of hydrogen bonding interactions that lie behind the enhanced uptake of one enantiomer over the other.
These results highlight the potential of non-3D coordination polymers in chemical separations and demonstrate the array of host-guest interactions that are required for separations of very similar compounds.
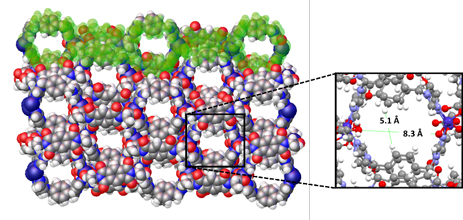
Figure 1. The chiral material contains guest-filled pores resulting from the alignment of loops within the 1D coordination polymer.
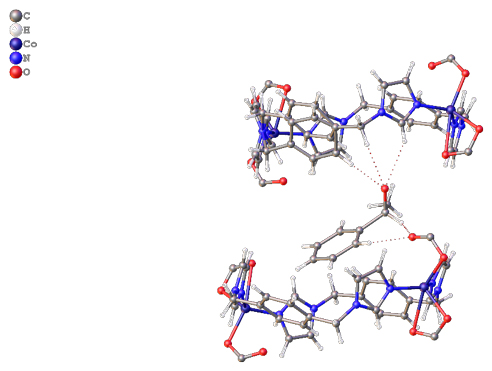
Figure 2. The preferred enantiomer of the guest is found crystallographically within the binding pocket, highlighting the array of interactions that hold it in place and provide the enantioselectivity.
Corresponding Author:
David Turner is a Senior Lecturer in the School of Chemistry at Monash University, Australia. After receiving his PhD in 2004 from King’s College, London, he held a number of fellowships at Monash University prior to joining as a Faculty member. Research in the Turner group revolves around metallosupramolecular assemblies, exploring both coordination polymers/MOFs and coordination cages with a particular emphasis on chirality. Dr Turner has published over 130 papers, in addition to two books, attracting almost 5000 citations and an h-index of 32.