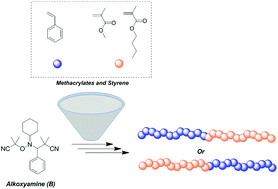
 Reversible addition-fragmentation chain transfer polymerization (RAFT) and transition metal-mediated radical polymerization (TMM-RDRP) are two widely used techniques employed for the preparation of controlled polymeric architectures. However, both of them exhibit significant colouring and complete purification of the final materials is challenging. On the other hand, nitroxide mediated polymerization (NMP) requires no or minimal purification although designing alkoxyamines that can facilitate the controlled polymerization of both styrene and methacrylates is a challenge. In this contribution, Asua and co-workers employed three different alkoxyamines to study the homopolymerization of styrene and its chain extension with methacrylates. Upon careful evaluation of the reaction kinetics as well as variation of the polymerization temperature, high monomer conversions with uncompromised end group fidelity could be achieved. A wide range of molecular weights were targeted in order to identify the limitations of the system. It was found that for targeted degrees of polymerization beyond 333, there was an increased difference between theoretical and experimental molecular weights, due to the thermal initiation of styrene. In addition, the nature of the substituents in the nitroxide adduct was also found to be crucial for the controlled polymerization of styrene with bulkier adducts providing more extensive control. The retention of the reactive alkoxyamine chain end was further confirmed via nuclear magnetic resonance (NMR) and matrix assisted laser desorption ionization time of flight mass spectrometry (MALDI-ToF-MS). Well-defined PS-b-PBMA and PMMA-b-PS block copolymers were also successfully prepared highlighting these classes of alkoxyamines as a versatile mediator for the controlled polymerization of both methacrylates and styrene.
Reversible addition-fragmentation chain transfer polymerization (RAFT) and transition metal-mediated radical polymerization (TMM-RDRP) are two widely used techniques employed for the preparation of controlled polymeric architectures. However, both of them exhibit significant colouring and complete purification of the final materials is challenging. On the other hand, nitroxide mediated polymerization (NMP) requires no or minimal purification although designing alkoxyamines that can facilitate the controlled polymerization of both styrene and methacrylates is a challenge. In this contribution, Asua and co-workers employed three different alkoxyamines to study the homopolymerization of styrene and its chain extension with methacrylates. Upon careful evaluation of the reaction kinetics as well as variation of the polymerization temperature, high monomer conversions with uncompromised end group fidelity could be achieved. A wide range of molecular weights were targeted in order to identify the limitations of the system. It was found that for targeted degrees of polymerization beyond 333, there was an increased difference between theoretical and experimental molecular weights, due to the thermal initiation of styrene. In addition, the nature of the substituents in the nitroxide adduct was also found to be crucial for the controlled polymerization of styrene with bulkier adducts providing more extensive control. The retention of the reactive alkoxyamine chain end was further confirmed via nuclear magnetic resonance (NMR) and matrix assisted laser desorption ionization time of flight mass spectrometry (MALDI-ToF-MS). Well-defined PS-b-PBMA and PMMA-b-PS block copolymers were also successfully prepared highlighting these classes of alkoxyamines as a versatile mediator for the controlled polymerization of both methacrylates and styrene.
Tips/comments directly from the authors:
- It is important to monitor the temperature throughout the polymerisation in order to ensure optimum kinetics.
- The appropriate temperature should be employed for methacylates (< 100 °C) and styrene (> 110 °C). For instance, this can influence the final molar mass distributions of a block copolymer. The preparation of a poly(methyl methacrylate) macro-alkoxyamine first, followed by the chain extension with styrene provides a PMMA-b-PS block copolymer with moderate MMD. However, the preparation of the PS block first would yield a block copolymer with high dispersity value due, in part, to the high temperatures employed.
- Several matrix/salt combinations should be tested when conducting MALDI-ToF MS analysis. The reactive chain ends might not be observed with some matrixes, such as trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile (DCTB).
Read this exciting research for free until 16/05/2017 through a registered RSC account:
Novel alkoxyamines for the successful controlled polymerization of styrene and methacrylates
Polym. Chem., 2017,8, 1728-1736, DOI: 10.1039/c6py02190e
—————-
Dr. Athina Anastasaki is a web writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please visit this website for more information.