Its bonds are constantly rearranging through a seemingly endless series of Cope rearrangements. It’s estimated that there are over 1.2 million possible valence tautomers, and due to the rapidity of the conversions, all carbons and protons appear as equivalent on the NMR timescale.
Professor Jeff Bode and his group at ETH Zürich have an interest in these fluxional molecules and their potential uses as chemical sensors.
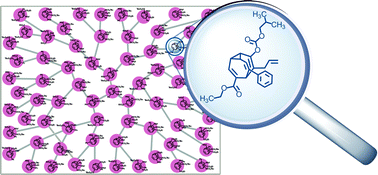
During a recent investigation into the racemisation of oligo-substituted members of the bullvalene family, they noticed some interesting behaviour in a sample of a tetrasubstituted bullvalene. A single isomer was spontaneously forming, and could be isolated from the mixture. This isomer was surprisingly stable, with a half-life of just over 4 hours at room temperature.
Now, tetrasubstituted bullvalenes have 1680 possible structural isomers and previous work has shown that the mixture is fully dynamic, so the appearance of just one isomer above all the others is pretty remarkable.
Their latest HOT PAPER builds on these results, and seeks to provide further information about the nature of this fascinating molecule.
After synthesis and isolation of the isomer (or “fraction e” as they call it, after its position on the HPLC trace), some very elegant NMR spectroscopic analysis has revealed its structure.
With a little computational assistance, Bode and co-workers have constructed a network diagram of all structural permutations of the molecule and the many, many Cope rearrangements that interconnect them. This reveals how many steps connect all the various isomers. Some further computational investigation has determined that the formation of “fraction e” is favoured by both kinetic and thermodynamic factors. They also discovered that the presence of an ester substituent increases the energy difference between the various structural isomers, so aids in the spontaneous resolution.
This extraordinary molecule, and the results presented in this paper, will inspire the design of future fluxional molecules for use in molecular sensors and smart materials.
E pluribus unum: isolation, structure determination, network analysis and DFT studies of a single metastable structure from a shapeshifting mixture of 852 bullvalene structural isomers
Maggie He and Jeffrey W. Bode
DOI: 10.1039/C2OB26954F
Published on behalf of Annabella Newton, Organic & Biomolecular Chemistry web science writer. Annabella Newton is a postdoctoral researcher based in Melbourne, Australia.










