Researchers in Canada have developed the first benzimidazolium-based synthetic ion channel and shown that it can cause damage to bacterial cells walls.
The movement of ions through cell walls is essential for a host of biological processes. The vast majority of this transportation occurs through ion channels or pores in the cell membrane. Man-made versions of these intracellular transport systems have been the subject of investigation by the supramolecular chemistry community for many years.
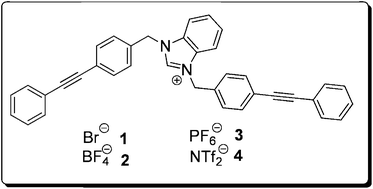 In this HOT paper, the Schmitzer group at the Université de Montréal are particularly interested in the transport of chloride ions in epithelial cells through calcium activated ion channels. They hope to mediate the concentrations of both calcium and chloride ions using synthetic ionophores.
In this HOT paper, the Schmitzer group at the Université de Montréal are particularly interested in the transport of chloride ions in epithelial cells through calcium activated ion channels. They hope to mediate the concentrations of both calcium and chloride ions using synthetic ionophores.
Building on their research into imidazolium amphiphiles, they have developed a benzimidazolium-based compound that increases chloride flux in a variety of lipid bilayer systems, and increases bacterial cell wall permeability to calcium ions.
This compound will hopefully inspire new drugs to treat cystic fibrosis, which is caused by mutation of a chloride-ion channel known as the cystic fibrosis transmembrane conductance regulator protein (CFTR). They may also aid in the development of new antibiotics to combat resistant strains of bacteria.
Want to find out more? Read this paper FREE for the next 4 weeks.
Benzimidazolium-based synthetic chloride and calcium transporters in bacterial membranes
Claude-Rosny Elie, Audrey Hébert, Mathieu Charbonneau, Adam Haiun and Andreea R. Schmitzer
DOI: 10.1039/C2OB26966J
Published on behalf of Annabella Newton, Organic & Biomolecular Chemistry web science writer. Annabella Newton is a postdoctoral researcher based in Melbourne, Australia.











![Synthesis of a four-component [3]catenane using three distinct noncovalent interactions](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C2OB26587G)




![1,5-(H, RO, RS) shift/6π-electrocyclic ring closure tandem processes on N-[(α-heterosubstituted)-2-tolyl]ketenimines](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C2OB27010B)