Metal-organic frameworks (MOFs) have become a recurring phenomenon in modern materials science. Every few years they re-enter the spotlight: first as the revolution for hydrogen storage, then as the future of CO₂ capture, then as the next big thing in catalysis, sensing, or drug delivery. And now once again, MOFs are appearing in discussions about the recent Nobel Prize.
A recent paper by Abánades Lázaro et al. published in Materials Horizons illustrates exactly why MOFs continue to generate excitement. Instead of commonly used strategy of adding a single functional group, they introduce two different modulators simultaneously during MOF synthesis. This is an example of multivariate MOF engineering, a major direction in recent MOF design. The authors present a clever strategy: introducing two types of modulators to create both functionality and controlled defects inside the classic UiO-66. This results in a 2.3× increase in CO₂ uptake. Mechanistically, two groups work cooperatively: SO₃⁻ introduces strong electrostatic interactions, and NH₂ interacts with the carbon atom of CO₂ through dipole -quadrupole interactions. This combination creates stronger binding sites around defect regions, which is confirmed by Grand Canonical Monte Carlo simulations.
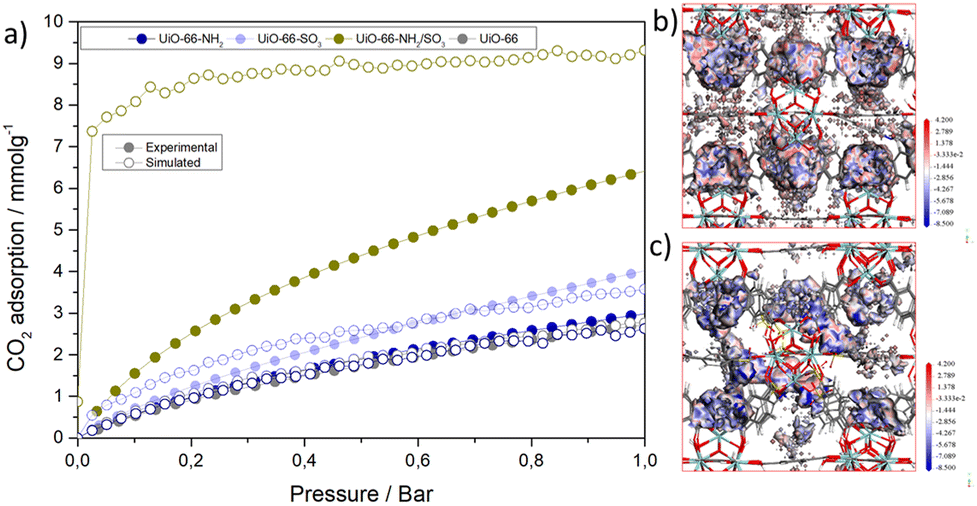
Figure 1 (a) Experimental and simulated CO2 adsorption isotherms at 273 K. CMC binding site of (b) UiO-66 and (c) UiO-66-NH2/SO3. Reproduced from DOI: 10.1039/D5MH00650C with permission from the Royal Society of Chemistry.
But is the excitement justified? Or is it yet another cycle of hype overshadowing the slow and complex path from elegant chemistry to industrial reality?
This paper perfectly captures the pattern:
Novel concept? Yes.
Better performance? Yes.
Elegant defect engineering? Absolutely.
Practical proof in real conditions? Not yet.
First, even though UiO-66 is among the cheaper MOFs, the added modulators, solvent usage, and synthesis complexity significantly increase the cost per kilogram. No large-scale industrial MOF adsorbent is commercially dominating the CO₂ market today. Secondly, 24 hours stability in many solvents (water, MeOH, MeCN, CHCl₃) is impressive but we still curious of tests for long-term cycling, high humidity CO₂ streams, industrial contaminants. And other questions about this new fascinating materials.
So, will it make MOFs competitive with amines, activated carbons, or zeolites for CO2 industry?
Not yet, but it pushes the field closer. We are at an important moment where solving the remaining challenges of stability and cost could finally transform MOFs from valuable laboratory materials into viable industrial solutions.
To find out more, please read:
Multivariate modulation of Zr6 UiO-66 for enhanced cooperative CO2 adsorption through defect multi-functionalisation
Carmen Rosales-Martínez, Sousa Javan Nikkhah, Marcileia Zanatta, Juan Carlos Martínez, Matthias Vandichel and Isabel Abánades Lázaro
Mater. Horiz., 2025,12, 5689-5693, DOI: 10.1039/D5MH00650C
About the blogger

Dr Olga Guselnikova is a member of the Materials Horizons Community Board. She joined the Center for Electrochemistry and Surface Technology (Austria) to work on functional materials as a group leader. Dr. Guselnikova received her PhD degree in chemistry from the University of Chemistry and Technology Prague (Czech Republic) and Tomsk Polytechnic University (Russia) in 2019. Her research interests are related to surface chemistry for functional materials. This means that she is applying her background in organic chemistry to materials science: plasmonic and polymer surfaces are hybridized with organic molecules to create high-performance elements and devices |