This month sees the following articles in MedChemComm that are in the top ten most accessed:
The developability of heteroaromatic and heteroaliphatic rings – do some have a better pedigree as potential drug molecules than others?
Timothy J. Ritchie, Simon J. F. Macdonald, Simon Peace, Stephen D. Pickett and Christopher N. Luscombe
Med. Chem. Commun., 2012, 3, 1062-1069
DOI: 10.1039/C2MD20111A
The use of phosphate bioisosteres in medicinal chemistry and chemical biology
Thomas S. Elliott, Aine Slowey, Yulin Ye and Stuart J. Conway
Med. Chem. Commun., 2012, 3, 735-751
DOI: 10.1039/C2MD20079A
Gd(III) chelates for MRI contrast agents: from high relaxivity to “smart”, from blood pool to blood–brain barrier permeable
Chang-Tong Yang and Kai-Hsiang Chuang
Med. Chem. Commun., 2012, 3, 552-565
DOI: 10.1039/C2MD00279E
Property based optimisation of glucokinase activators – discovery of the phase IIb clinical candidate AZD1656
Michael J. Waring, David S. Clarke, Mark D. Fenwick, Linda Godfrey, Sam D. Groombridge, Craig Johnstone, Darren McKerrecher, Kurt G. Pike, John W. Rayner, Graeme R. Robb and Ingrid Wilson
Med. Chem. Commun., 2012, 3, 1077-1081
DOI: 10.1039/C2MD20077E
A matched molecular pair analysis of in vitro human microsomal metabolic stability measurements for heterocyclic replacements of di-substituted benzene containing compounds – identification of those isosteres more likely to have beneficial effects
Alexander G. Dossetter, Adam Douglas and Charles O’Donnell
Med. Chem. Commun., 2012, 3, 1164-1169
DOI: 10.1039/C2MD20155K
Small molecules targeting phosphoinositide 3-kinases
Peng Wu and Yongzhou Hu
Med. Chem. Commun., 2012, Advance Article
DOI: 10.1039/C2MD20044A
Minisci reactions: Versatile CH-functionalizations for medicinal chemists
Matthew A. J. Duncton
Med. Chem. Commun., 2011, 2, 1135-1161
DOI: 10.1039/C1MD00134E
On the importance of synthetic organic chemistry in drug discovery: reflections on the discovery of antidiabetic agent ertugliflozin
Vincent Mascitti, Benjamin A. Thuma, Aaron C. Smith, Ralph P. Robinson, Thomas Brandt, Amit S. Kalgutkar, Tristan S. Maurer, Brian Samas and Raman Sharma
Med. Chem. Commun., 2012, Advance Article
DOI: 10.1039/C2MD20163A
Optimisation of aqueous solubility in a series of G protein coupled receptor 119 (GPR119) agonists
James S. Scott, Alan M. Birch, Katy J. Brocklehurst, Hayley S. Brown, Kristin Goldberg, Sam D. Groombridge, Julian A. Hudson, Andrew G. Leach, Philip A. MacFaul, Darren McKerrecher, Ruth Poultney, Paul Schofield and Per H. Svensson
Med. Chem. Commun., 2012, Advance Article
DOI: 10.1039/C2MD20130E
Editorial: natural products themed issue
Sylvie Garneau-Tsodikova and Christopher T. Walsh
Med. Chem. Commun., 2012, 3, 852-853
DOI: 10.1039/C2MD90031A
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to MedChemComm? Then why not submit to us today or alternatively email us your suggestions.






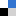





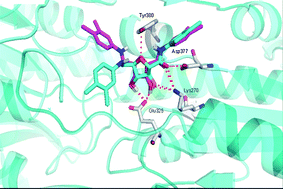 Human African trypanosomiasis, also commonly known as sleeping sickness, is a disease that leads to many deaths worldwide and is caused by protozoan parasites. When in the bloodstream these parasites can only produce the ATP (adenosine triphosphate) they need to survive via a glycolytic pathway, making this pathway essential for the parasite’s survival.
Human African trypanosomiasis, also commonly known as sleeping sickness, is a disease that leads to many deaths worldwide and is caused by protozoan parasites. When in the bloodstream these parasites can only produce the ATP (adenosine triphosphate) they need to survive via a glycolytic pathway, making this pathway essential for the parasite’s survival.



 Antimalarial drugs with increased in vitro
Antimalarial drugs with increased in vitro