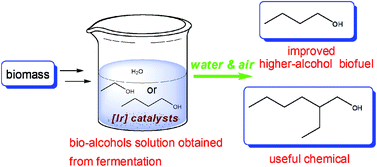
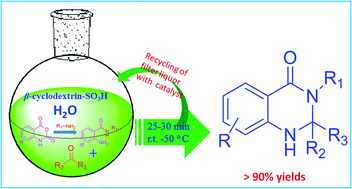
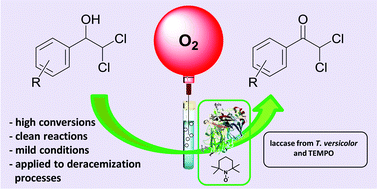
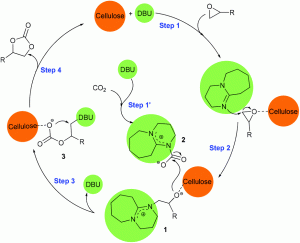
 A wide variety of metal complexes act as efficient catalysts for the synthesis of cyclic carbonates from carbon dioxide and epoxides; organic bases, such as pyridine, are often useful co-catalysts in the reaction. Metal-free catalyst systems are also effective, and polymers having abundant hydroxyl groups, such as cellulose, are known to catalyze cycloaddition when combined with an alkali metal halide. Building upon these findings, researchers from the Chinese Academy of Sciences have developed a metal-free and halide-free catalyst system using a combination of a superbase and a hydrogen bond donor.
A wide variety of metal complexes act as efficient catalysts for the synthesis of cyclic carbonates from carbon dioxide and epoxides; organic bases, such as pyridine, are often useful co-catalysts in the reaction. Metal-free catalyst systems are also effective, and polymers having abundant hydroxyl groups, such as cellulose, are known to catalyze cycloaddition when combined with an alkali metal halide. Building upon these findings, researchers from the Chinese Academy of Sciences have developed a metal-free and halide-free catalyst system using a combination of a superbase and a hydrogen bond donor.
Among the bases and hydrogen bond donors investigated, a cellulose-DBU catalyst system exhibited the highest conversion to propylene carbonate. Optimization of the reaction conditions led to a further study using an array of terminal epoxides; the highest yield and selectivity was observed for ethylene oxide, with lower yields for more sterically hindered substrates. The catalyst system also proved to be recyclable for up to four trials without an appreciable loss of activity or selectivity.
Read the full article now:
Superbase/cellulose: an environmentally benign catalyst for chemical fixation of carbon dioxide into cyclic carbonates
Jian Sun, Weiguo Cheng, Zifeng Yang, Jinquan Wang, Tingting Xu, Jiayu Xin and Suojiang Zhang
Green Chem. 2014, Advance Article, DOI: 10.1039/C3GC41850B
 Jenna Flogeras obtained her B.Sc. and M.Sc. in Chemistry from the University of New Brunswick (Fredericton), Canada. Currently a Ph.D. student at Memorial University of Newfoundland, she is excited to spend some time outside the laboratory this summer to explore Thailand and Southeast Asia.
Jenna Flogeras obtained her B.Sc. and M.Sc. in Chemistry from the University of New Brunswick (Fredericton), Canada. Currently a Ph.D. student at Memorial University of Newfoundland, she is excited to spend some time outside the laboratory this summer to explore Thailand and Southeast Asia.
 Scientists in Canada and China have shown that the effectiveness of commercial sunscreens can be enhanced by the addition of lignin and, as an unprecedented bonus; sunlight exposure may help them work even better!
Scientists in Canada and China have shown that the effectiveness of commercial sunscreens can be enhanced by the addition of lignin and, as an unprecedented bonus; sunlight exposure may help them work even better!