Humic substances play an important role in the speciation of metal ions in the aquatic environment. Understanding these processes is important to ensure the safety of drinking water. In this study, Japanese researchers reveal how physiochemical properties and ion-binding behaviour of HSs can differ between surface water and groundwater environments.
Groundwater constitutes the largest reservoir of freshwater in the world, accounting for over 97% (excluding glaciers and ice caps) of all available freshwater on the planet. It is estimated that about 75% of EU inhabitants depend on groundwater for their water supply. Groundwater also plays an integral role in the hydrological cycle, crucial to the maintenance of wetlands and river flows. Maintaining the quality of groundwater resources is therefore of major environmental, social and economic importance. This requires a good understanding of the physical and chemical processes that may influence groundwater environments.
For future use of deep underground space it is necessary to monitor and protect the quality of deep groundwater. The World Health Organisation (WHO) acknowledges the importance of groundwater resources and the potential risks that poor quality groundwater can cause to human health. Indeed, the WHO has produced a guidance document, “analysing hazards to groundwater quality, assessing the risk they may cause for a specific supply, setting priorities in addressing these, and developing management strategies for their control.”
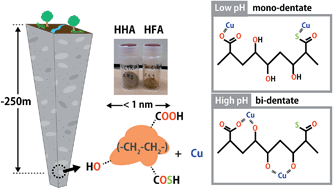
Humic substances (HSs) are a class of natural organic molecules, ubiquitous in various environments including surface and ground water, oceans, soils, and the atmosphere. HSs are known to play an important role in freshwater systems. For example HSs can effectively capture inorganic and organic contaminants due to the tendency of protons and metal ions to readily bind to the functional groups of HS ligands e.g., carboxylic and phenolic groups and to a lesser extent amine- and sulphur-containing groups. This process can therefore alter the reactivity, bioavailability, and mobility of chemical constituents in fresh water.
The physical and chemical nature of HSs is likely to differ between deep underground and surface aquatic systems, due to slower water movement and more prolonged contact with underlying rocks and dissolved/suspended components, low oxygen, and lack of sunlight. However, while some characteristics of groundwater HSs are understood, their ion binding properties over a wide range of conditions is largely unknown. Given that in many areas, deep underground space may be used in the future for such uses as geological disposal of nuclear wastes, the potential deterioration of groundwater quality, the ion binding properties of deep groundwater HSs need to be studied carefully, and mechanistic models developed to describe these processes.
This study by Takumi Saito and co-workers investigates the physicochemical and binding properties of HSs isolated from deep groundwater in a sedimentary rock formation in the Horonobe Underground Research Laboratory of the Japan Atomic Energy Agency (JAEA). Binding isotherms of protons (H+) and copper (Cu2+) were measured over a wide range of conditions by potentiometric titration and were fitted to the NICA-Donnan model and the obtained parameters were compared with average parameters for HSs from surface environments. The oxidation state and local coordination environment of Cu2+ bound to the HA fraction of the HSs were also assessed by X-ray absorption spectroscopy (XAS).
The results clearly indicate distinctive physical and chemical characteristics for the HSs from surface and groundwater environments. It is found that the deep ground HSs were characterised by high aliphaticity and sulphur (S) content and relatively small sizes. Differences in the binding behaviours of H+ and Cu2+ with the HSs in deepwater and surface water were also observed.
The authors discuss the differences in chemical binding behaviour with reference to the unique chemical nature of the functional groups of groundwater HSs compared with those of surface waters and how the binding sites and binding mode can change with changes in conditions (e.g. pH). X-ray absorption spectroscopy also revealed that Cu2+ binds to O/N containing functional groups and to a lesser extent S containing functional groups.
The study therefore demonstrates how HSs could influence freshwater resources differently in deep-ground and surface environments. Although the HSs in this study were derived from a single groundwater source, the authors suggest the outcomes can be applied or be a good starting point to estimate the degree of metal binding to HSs in sedimentary groundwater in general.
The authors also suggest these results should be examined in the future by performing similar investigations for HSs isolated from a range of different groundwater samples and for a wider range of metal ions. This would allow accurate and realistic parameter sets applicable for groundwater HSs to be developed for further modelling for a more extensive range of chemical species.
To read more about this research, download a copy of the manuscript for free* by clicking the link below.
Physicochemical and ion-binding properties of highly aliphatic humic substances extracted from deep sedimentary groundwater
Takumi Saito, Motoki Terashima, Noboru Aoyagi, Seiya Nagao, Nobuhide Fujitake and Toshihiko Ohnuki
Environ. Sci.: Processes Impacts, 2015,17, 1386-1395
DOI: 10.1039/C5EM00176E
—————-

About the webwriter
Ian Keyte is a Doctoral Researcher at the University of Birmingham. His research focuses on the sources, behavior and fate of polycyclic aromatic hydrocarbons (PAHs) in the atmosphere.
—————-
* Access is free until 20/09/2015 through a registered RSC account.