Scientists from Korea have found that with the use of graphene nanosheets, the fabrication of bendable power sources is possible.
Electronic devices are no longer confined to the home or office. We travel with them, carry them around and even wear them. To make equipment like roll-up displays and wearable devices achievable, the power source that supplies them must also become more flexible.
The major challenge of developing a truly bendable power source has been the shortage of material that is both highly flexible and has superior electronic conductivity. Polymers are typically used, but they can degrade at relatively low temperatures, which makes them less than ideal.
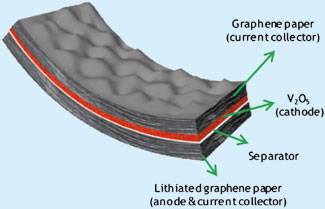
Kisuk Kang from the Korea Advanced Institute of Science and Technology in Daejon, and colleagues, have developed a graphene based hybrid electrode producing a flexible lithium rechargeable battery. The cathode material, in this case V2O5, is grown on graphene paper using pulsed laser deposition and graphene paper coated in lithium is used as the anode. The resultant battery is lightweight and flexible enough to be twisted or rolled.
Want to find out more?
Read the rest of the Chemistry World story by Rebecca Brodie
Or view the Energy & Environmental Science article in full:
Flexible energy storage devices based on graphene paper
Hyeokjo Gwon, Hyun-Suk Kim, Kye Ung Lee, Dong-Hwa Seo, Yun Chang Park, Yun-Sung Lee, Byung Tae Ahn and Kisuk Kang,
Energy Environ. Sci., 2011, DOI: 10.1039/c0ee00640h











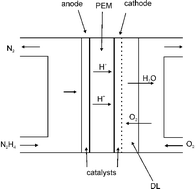 Reviewing recent advances in ammonia and hydrazine based electrochemical fuel cells
Reviewing recent advances in ammonia and hydrazine based electrochemical fuel cells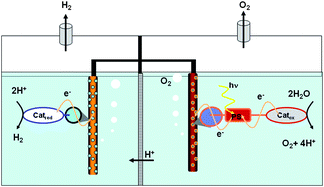 This review covers the progress achieved in the synthesis and characterization of different metal based catalysts designed for the photocatalytic oxidation of water, with special focus on molecular designed systems.
This review covers the progress achieved in the synthesis and characterization of different metal based catalysts designed for the photocatalytic oxidation of water, with special focus on molecular designed systems.