Since their first publication, zwitterionic benzo quinonemonoimines such as have attracted much attention, because of the remarkable electronic delocalization of their pi-system which forms two chemically connected but electronically separated 6pi-electron subunits. Their potential antiaromaticity is of theoretical interest, and the unique properties that such ligands may confer to their metal complexes extend to coordination chemistry, optical recording, homogenous catalysis and supramolecular chemistry.
In this Dalton Transactions Hot Article, Thomas Kauf and Pierre Braunstein compare the properties of TCNE and TCNQ zwitterionic benzoquinonemonoimine derivatives to find some interesting results. Be the first to read their communication here – free for you to view for 4 weeks:
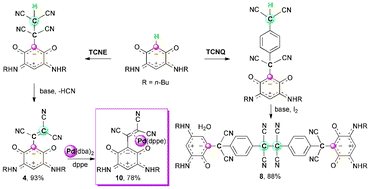 Contrasting behaviour of TCNE and TCNQ zwitterionic benzoquinonemonoimine derivatives and coordination of a tricyanoethenyl substituent to Pd(0)
Contrasting behaviour of TCNE and TCNQ zwitterionic benzoquinonemonoimine derivatives and coordination of a tricyanoethenyl substituent to Pd(0)
Thomas Kauf and Pierre Braunstein
Dalton Trans., 2011, Advance Article
DOI: 10.1039/C1DT10804B










