Sara Coles is a guest web-writer for Catalysis Science & Technology. She currently works for Johnson Matthey in Royston, UK.
Gold-palladium nanoparticles are hot stuff when it comes to catalysis. Catalysis Science and Technology’s themed issue, entitled ‘Gold Catalysis’, highlights just three examples of the many studies that regularly appear in the literature.
A minireview from Pasi Paalanen et al., Utrecht University, gives an overview of recent developments in the synthesis of supported gold-based bimetallic nanoparticles for catalytic applications. They focus on three major structural features to be characterised and, where possible, controlled: size, composition and nanostructure. They highlight selected literature examples in which gold-palladium nanoparticles were found to be active for reactions such as CO oxidation, vinyl acetate synthesis, cyclotrimerization of acetylene to benzene, selective oxidation of alcohols to aldehydes or ketones, direct synthesis of hydrogen peroxide, hydrocarbon hydrogenation, oxidation of primary C–H bonds, hydrodechlorination and hydrodesulfurization.
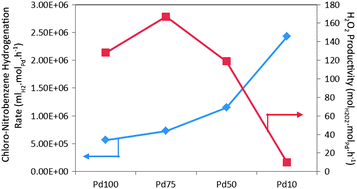
Tatsumi Ishihara et al., Kyushu University, Japan, carried out synthesis of hydrogen peroxide by direct oxidation of hydrogen in air on gold-palladium/titania. They report that the H2 conversion and H2O2 selectivity were strongly affected by the crystal phase of the titania. With increasing H2 pressure, H2O2 selectivity increased on AuPd/rutile TiO2 and the yield of became higher than on brookite or anatase TiO2 at 1.0 MPa. The effects of fluorinated hydrocarbon addition to reaction media were also studied.
Find out more about all this research in Catalysis Science and Technology.
Progress in controlling the size, composition and nanostructure of supported gold–palladium nanoparticles for catalytic applications
Pasi Paalanen, Bert M. Weckhuysen and Meenakshisundaram Sankar
Catal. Sci. Technol., 2013,3, 2869-2880, DOI: 10.1039/C3CY00341H
Tuning the properties of PdAu bimetallic nanocatalysts for selective hydrogenation reactions
Elena C. Corbos, Peter R. Ellis, James Cookson, Valérie Briois, Timothy I. Hyde, Gopinathan Sankar and Peter T. Bishop
Catal. Sci. Technol., 2013,3, 2934-2943, DOI: 10.1039/C3CY00255A
Effects of fluorinated hydrocarbon addition on H2O2 direct synthesis from H2 and air over an Au–Pd bimetallic catalyst supported on rutile-TiO2
Tatsumi Ishihara, Kohei Shigeta, Yuuki Ooishi, Maki Matsuka, Hidehisa Hagiwara and Shintaro Ida
Catal. Sci. Technol., 2013,3, 2971-2975, DOI: 10.1039/C3CY00273J











