I know I’ve highlighted articles on fullerenes before in this blog, and although I try not to repeat myself too often, the ever evolving story of C60 is a topic too interesting to stay away from. This time around I’ve been learning about the extra special properties of anionic C60, as recently described by Klaimen and co-authors.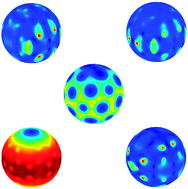
One of the most exciting things about the C60 anion is that it has electronically bound excited states, a property not shared by less exciting molecules of similar electron affinity. In this paper, the authors delve into the all-important, but hitherto unanswered, question of why it is that these excited states are able to exist. In order to do so, they turn to high level theory, which allows them to consider the full electron densities of both the anion and the neutral molecule in order to gain their new insight.
The conclusion the authors reach is simultaneously important, neat and potentially liable to cause controversy. If that prospect piques your interest, then I suggest you go and read the article to find out all the details, as they’re best read in the authors’ own words.
By Victoria Parkes, Guest web writer based at the University of Nottingham
Read the full article in PCCP here: All for one and one for all: accommodating an extra electron in C60
Shachar Klaiman, Evgeniy V. Gromov and Lorenz S. Cederbaum
Phys. Chem. Chem. Phys., 2014, 16, 13287–13293