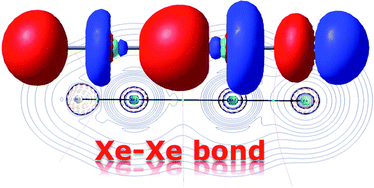
Fernández and Frenking conducted a theoretical investigation into the stability and nature of the bonding involved in two classes of compounds containing a Xe-Xe bond. No species from either of the two groups, HXeXeX (where X=halogen) and RXeXeR’ (where R and R’ are both halogens), have ever been observed experimentally, and based on their results, several compounds for which this might be possible were identified.
The strong correlation observed between Xe-Xe bond length and the energy barrier to decomposition suggests that the strength of this bond plays a vital role in the stability of the molecule. Both of these characteristics were shown to decrease with increasing halogen mass, and generally indicated stronger bonding and greater stability in the RXeXeR’ molecules compared with HXeXeX. On this basis, HXeXeF and FXeXeF were proposed as the most viable compounds for experimental isolation.
The former was shown to be the result of two electrostatically bonded fragments, HXeXe+ –F, with a strong covalent H-Xe bond. The positive charge is shared over the two Xe atoms, with the smaller proportion on the Xe next to the F atom. The Xe-Xe bond in this case is thought to be primarily electrostatic, and involve only the px orbital. By analogy, FXeXeF could either be formed of similar electrostatically bonded fragments, or through the interaction of two FXe· radicals. The calculations pointed to contributions from both mechanisms, with a small but significant bias toward radical interaction.
This research represents another step in unravelling and understanding the complicated and often surprising chemistry of the noble gases. It would therefore be marvellous to see experimental confirmation of their findings in the future.
By Victoria Wilton
Read the full details of this fascinating article which was published as part of the PCCP themed issue on Predicting new molecules by quantum chemical methods:
Neutral noble gas compounds exhibiting a Xe–Xe bond: structure, stability and bonding situation
Israel Fernández and Gernot Frenking
DOI: 10.1039/C2CP41244F