Antibiotic resistant bacteria, and the subsequent diseases caused by their infection, are of serious global concern. As more and more bacteria develop antibiotic resistance and jeopardise the current treatments for serious infections, there is a strong imperative to both develop new medicines and to understand these bacterial pathogens. Pseudomonas aeruginosa, Staphylococcus aureus and species from the genus Burkholderia are all such antibiotic resistant bacteria that contribute to various human diseases, and they can pose a serious threat to cystic fibrosis patients through chronic lung infections. These are often polymicrobial infections, meaning that the different bacteria interact by association and can alter the impact of the resulting disease.
P. aeruginosa and Burkholderia generally interact competitively with S. aureus, reducing its viability. This is achieved by the secretion of small molecule respiratory toxins which include 2-alkyl-4(1H)-quinolone N-oxides (AQNOs) by P. aeruginosa or 3-methyl-2-alkyl-4-quinolone N-oxides (MAQNOs) by Burkholderia (see Figure 1). Researchers in Germany and Austria sought to understand the antagonistic interactions of these bacteria and have now reported the synthesis of various representative AQNOs and MAQNOs and investigated their action against S. aureus.
Figure 1: Structures of the quinolone derivatives produced by P. aeruginosa and Burkholderia that act against S. aureus
The researchers approached the synthesis of the AQNOs and MAQNOs by starting with the preparation of the corresponding quinolones, and then converting them to the quinolone N-oxides. They focussed on the C9 nonyl-/nonenyl- derivatives, NQNOs and MNQNOs, as previous studies showed this alkyl chain length proved the most active against S. aureus. Mass spectrometry and fragmentation was primarily used to characterise the synthesised compounds, and the researchers were able to establish a new library of standards to be used for the identification of quinolones and quinolone N-oxides. This therefore allowed the researchers to quantify the specific quinolone derivatives produced by certain strains of P. aeruginosa and Burkholderia using this standard library, as shown in Figure 2.
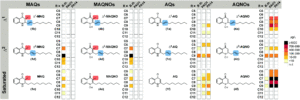
Figure 2: Quantification of the quinolones (AQs and MAQs) and quinolone N-oxides (AQNOs and MAQNOs) secreted by P. aeruginosa (strains PAO1 and PA14) and Burkholderia thailandesis using calibration against the established standard library
The researchers then investigated the possible activity of these quinolone derivatives against S. aureus. The activity of S. aureus was measured using a chromogenic assay, by varying concentrations of the quinolone derivatives until a minimum inhibitory concentration (MIC) was reached, with complete respiratory inhibition of the bacteria. The C9-quinolones (before N-oxidation) showed no inhibition against S. aureus at the highest concentrations tested, but the corresponding quinolone N-oxides (NQNOs and MNQNOs) showed activity against the bacteria. More specifically, unsaturated derivatives were more active, and the MNQNOs, with 3-methylation of the quinolone core, showed the greatest antibiotic activity against S. aureus. These results suggest that the methylated quinolones produced by species of Burkholderia, as well as unsaturared quinolones produced by P. aeruginosa, have an important role in competitive interactions against S. aureus in polymicrobial infections.
To find out more, please read:
Profiling structural diversity and activity of 2-alkyl-4(1H)-quinolone N-oxides of Pseudomonas and Burkholderia
Dávid Szamosvári, Michaela Prothiwa, Cora Lisbeth Dieterich and Thomas Böttcher
Chem. Commun., 2020, 56, 6328-6331
About the blogger:
 Dr. Samantha Apps is a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.
Dr. Samantha Apps is a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.










