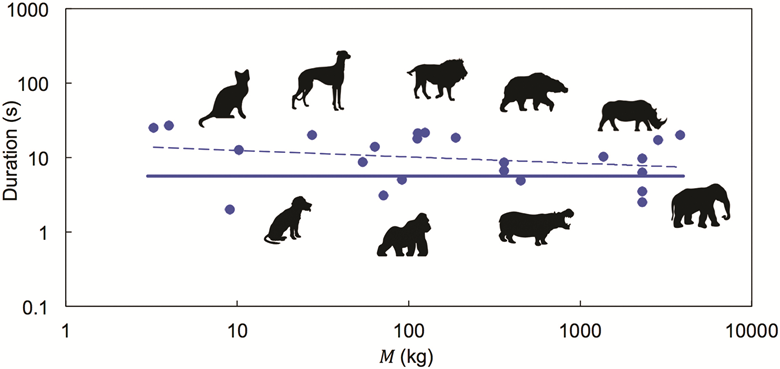
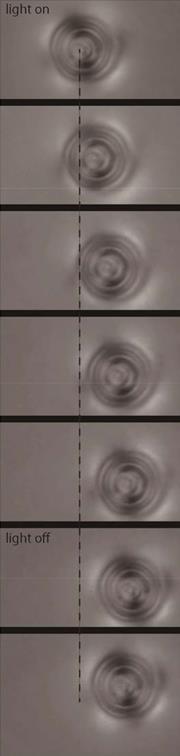
From a 4kg cat to a 4000kg elephant, any animal takes only around 12 seconds to ‘do their business’. This is what a team of US scientists found after thoroughly studying the process of defecation in over 40 animal species. They then developed a theoretical fluid dynamics model that shows how viscosity changes in faecal mucus ease excretion.
Despite being extensively studied from a clinical and medical perspective, the physics of pooing has until now escaped extensive exploration.
Inspired by a slow motion video of a defecating elephant obtained during their previous work on urination, David Hu and his team from Georgia Institute of Technology acquired similar videos of other mammals ranging from dogs to giant pandas. They found that despite obvious differences in animals’ size and diet, defecation time averaged to around 12 (±7) seconds across all of the species they looked at.
Read the full story by Charlie Quigg in Chemistry World.
This article is free to access until 21 June 2017
P J Yang et al, Soft Matter, 2017, DOI: 10.1039/C6SM02795D