As children, we learn very early on about the concept of shape and size complementarity. No matter how many times you try, the square peg doesn’t fit in the round hole, and that second (or third!) piece of cake just wasn’t a good idea. This same concept also extends to supramolecular interactions, especially when we consider the arena of host–guest chemistry.
Generally speaking, the conformation of a macrocyclic host is relatively rigid, which means that the scope of host molecules it can encase is also somewhat limited. Whilst this feature of host–guest chemistry and molecular recognition is the basis for a range of catalytic events and the template-directed synthesis of mechanically interlocked molecules, it would be advantageous in expanding the scope of this field if a macrocycle existed that could happily host a wide range of guest molecules.
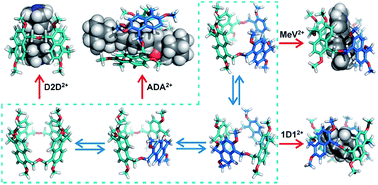
The dynamic nature of this unique macrocycle is an important step forward in the construction of host–guest complexes, especially as we look to introduce further complexity into the arsenal of supramolecular interactions we have at our disposal, and particularly in the development of increasingly multifaceted stimuli-responsive and molecular machines.
Read this hot ChemSci article in full – it’s open access and free to download:
Oxatub[4]arene: A smart macrocyclic receptor with multiple interconvertible cavities
Fei Jia, Zhenfeng He, Liu-Pan Yang, Zhi-Sheng Pan, Min Yi, Ren-Wang Jiang and Wei Jiang
Chem. Sci., 2015, Advance Article.
DOI: 10.1039/C5SC03251B, Edge Article
 About the Writer:
About the Writer:
Anthea Blackburn is a guest web writer for Chemical Science. Anthea is a recent graduate student hailing from New Zealand. She studied at Northwestern University in the US under the tutelage of Prof. Fraser Stoddart (a Scot), where she exploited supramolecular chemistry to develop multidimensional systems and study the emergent properties that arise in these superstructures. When time and money allowed, she ambitiously attempted to visit all 50 US states.












![The binding of calix[4]pyrrole to guests immobolised on a surface is, kinetically, slower than in bulk solution](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C4SC01745E)