Surface runoff of agricultural or landscape areas with excessive nitrate fertilizers has resulted in increasing nitrate ion concentrations in freshwater. This issue leads to eutrophication and algae blooms that significantly threaten aquatic lives and ecosystems. Eliminating nitrate ions is thus necessary to address the nitrate-borne water pollution. Nature presents a delicate yet complicated process for turning NO3– into N2 by four metalloenzymes. Is it possible to develop an artificial method with just one catalyst to drive the same process?
Lee, Baik, and coworkers at the Institute for Basic Science (IBS) and Korea Advanced Institute of Science and Technology (KAIST) answered yes. The researchers synthesized a square-planar nickel(II)-based complex, (PNP)Ni(ONO2) (PNP = N[2-P’Pr-4-methyl-C6H3]2), which converted NO3– to N2 in the presence of CO and NO. Ni2+ is the only active site. This breakthrough has been published in Chemical Science (DOI: 10.1039/C9SC00717B).
The reaction mechanism involves four successive redox reactions among (PNP)Ni(ONO2), CO, and NO (Figure 1). The first two steps consecutively transfer two oxygen atoms from (PNP)Ni(ONO2) to CO at room temperature, yielding two molecules of CO2 and a Ni-nitrosyl complex, (PNP)Ni(NO). Afterwards, the (PNP)Ni(NO) undergoes a disproportionation reaction with NO, generating (PNP)Ni(NO2) and N2O. The as-formed N2O interacts with the remaining (PNP)Ni(NO) and is eventually reduced to N2. The yield of N2 is 46% (based on the amount of N2O).
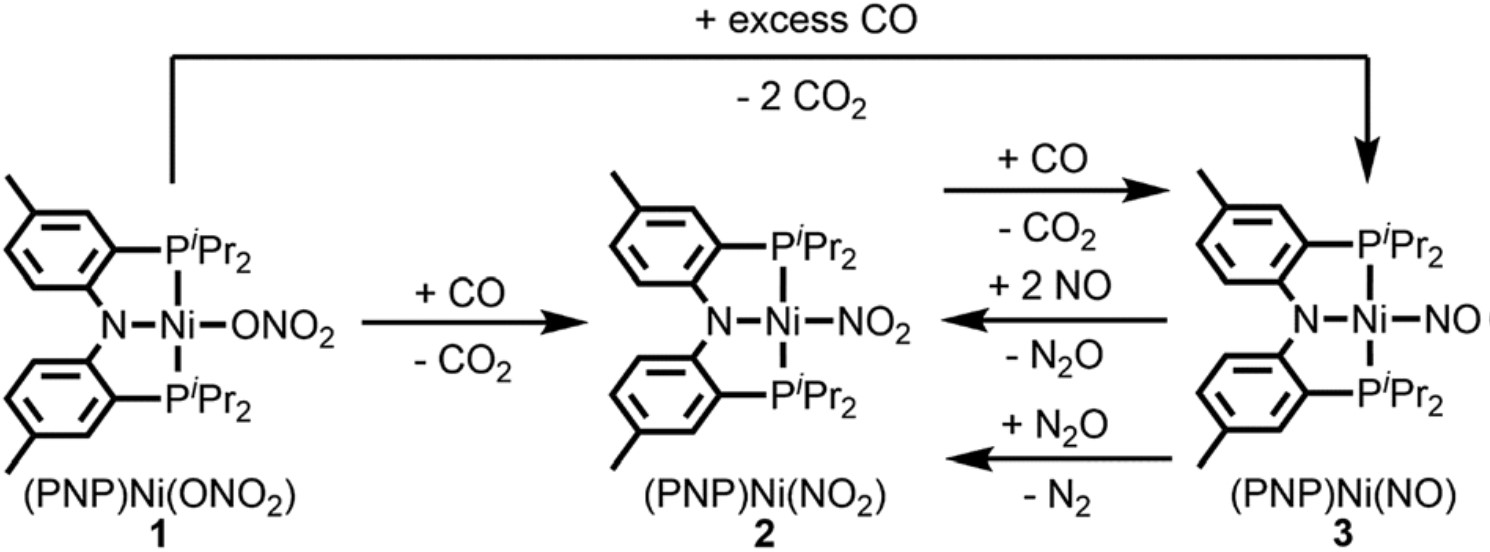
Figure 1. The schematic shows the artificial nitrate reduction with a Ni(II)-based complex, (PNP)Ni(ONO2), as the catalyst. Pr: propyl group.
Besides nitrate reduction, the (PNP)Ni(ONO2) could act as a potential alternative to the platinum-containing catalysts used in the catalytic converters of gasoline cars, because it transforms hazardous CO and NO into less toxic CO2 and N2.
To find out more please read:
One Metal is Enough: A Nickel Complex Reduces Nitrate Anions to Nitrogen Gas
Jinseong Gwak, Seihwan Ahn, Mu-Hyun Baik and Yunho Lee
Chem. Sci., 2019, 10, 4767-4774
About the blogger:
 Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.
Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.










