As chemists, we typically first encounter molecular switches, the general term for any molecule that exists in at least two stable or meta-stable states, as pH indicators in introductory chemistry classes. The external factor that causes the conversion can vary from a redox event to UV light. This makes molecular switches attractive sensors for a range of chemically relevant applications. The reversible and controllable bond-making and bond-breaking also provides a system within which to interrogate the nature of chemical bonding.
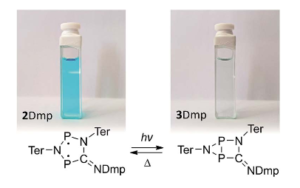
Figure 1. The biradical (left) and housane (right) in solution along with their simplified chemical structures.
Chemists at the University of Rostock in Germany explored light-driven molecular switching behavior in a class of heterocyclic molecules. These can exist as either biradicals or housanes (see Figure 1 for why the name makes sense) with a bond between the two phosphorus atoms. Understanding the mechanism by which the photo-isomerization occurs is important for future applications of the biradical system in small molecule activation. The in-depth studies used the 2,6-dimethylphenyl (2Dmp) derivative, since it was the most stable of the synthesized derivatives. The researchers monitored the conversion between species by examining their dramatically different 31P NMR spectra, with the housane resonances between -50 and -200 ppm and the biradical resonances between 200 and 300 ppm. An additional distinguishing feature between is color: the starting biradical is blue while the housane is colorless. The researchers found that biradical converts to the housane, which has a half-life of about 7 minutes at room temperature in solution, with an almost 25% quantum yield.

Figure 2. A single crystal of the biradical. Upon irradiation, the transition to the colorless housane can be observed, along with extensive cracking of the crystal.
This switching occurs not only in solution, but also in the solid state (Figure 2). The cracking evident in the images of a single crystal is attributed to the stress caused by changes to the crystal lattice by conversion of the biradical to the housane. Despite various efforts by the team, including crystallization attempts under constant irradiation, they were unable to obtain high enough quality crystals to acquire a crystal structure of the housane. The specific isomerization mechanism was computationally modeled and showed that the photoexcitation of the biradical led to a bonding interaction and distortion, allowing for housane formation. The reverse, thermally activated process, occurs due to the intersection of the ground and excited state energies near a transition state.
Given this enhanced understanding of the isomerization mechanism, the researchers manipulated the reactivity of the biradical by adding tert-butyl isocyanide (tBuNC). tBuNC catalyzed the thermal conversion of the housane back to the diradical. While the mechanism of the catalysis is unknown, it’s an exciting proof-of-concept for easily tuning molecular switching behavior.
To find out more please read:
Jonas Bresien, Thomas Kröger-Badge, Stefan Lochbrunner, Dirk Michalik, Henrik Müller, Axel Schultz, and Edgar Zander
Chem. Sci., 2019, 10, 3486-3493
About the blogger:
Beth Mundy is a PhD candidate in chemistry in the Cossairt lab at the University of Washington in Seattle, Washington. Her research focuses on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.











