Reducing CO2 into fuels such as CO or hydrocarbons is a promising strategy to reduce the net emission of greenhouse gases and mitigate climate change. The CO2 reduction reaction, however, is an energy-costly process. Therefore, CO2 reduction catalysts are necessary to enhance the CO2 conversion efficiency and minimize the overall energy demand.
Researchers are developing high-performance yet inexpensive CO2 reduction catalysts. Noble metals such as Au, Ag and Pd exhibit high CO2-to-CO conversion efficiency, but their scarcity restricts their large-scale practicability. Metallic Fe, Co and Ni are active in reducing CO2 and therefore, have been identified as alternatives to the noble metal catalysts.
Recently in Chem. Sci., a group of scientists led by Zhenyu Sun from Beijing University of Chemical Technology and Yousung Jung from Korea Advanced Institute of Science and Technology (KAIST) pushed the CO2-reduction activity of Ni metal to a new height. The researchers synthesized carbon-supported Ni nanocrystals via pyrolysis of Ni-based metal organic frameworks (MOFs) in argon. The resultant Ni nanoparticles had an average diameter of ~30 nm and were embedded in N-doped carbon scaffolds (Fig. 1a). Each nanoparticle was uniformly coated with a thin layer of amorphous carbon (Fig. 1b).
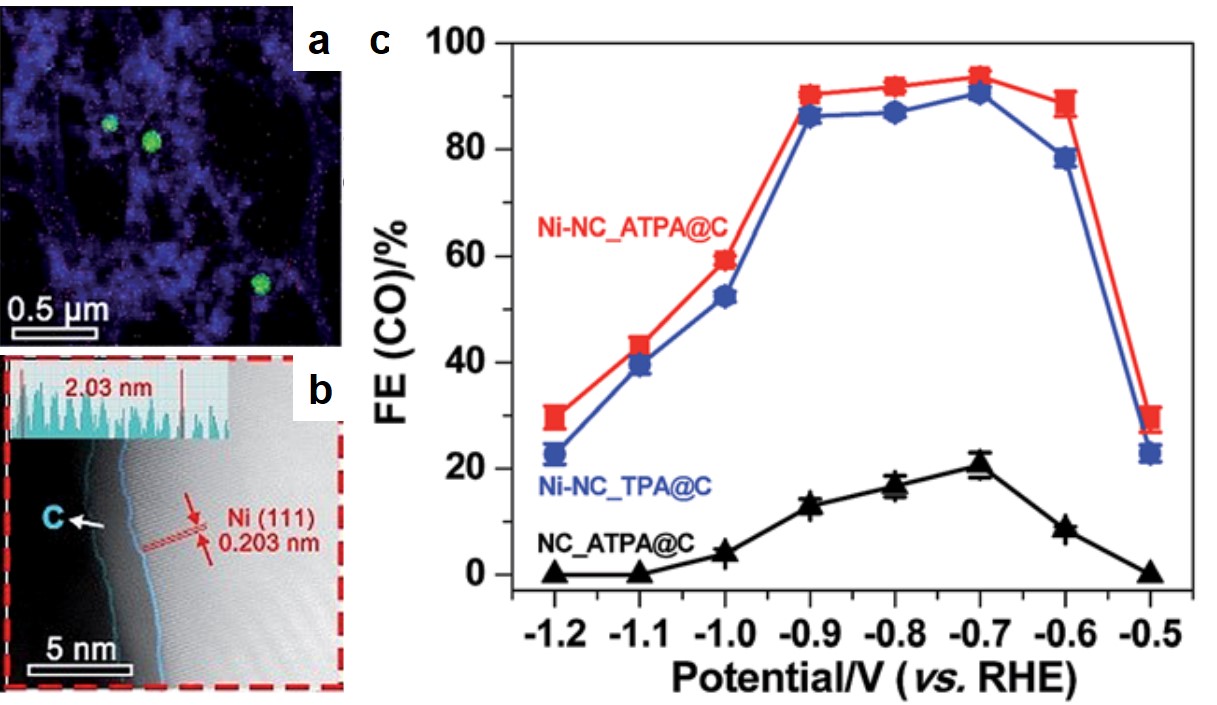
Figure 1. (a) The elemental mapping of the carbon-supported Ni nanoparticles. Green dots and blue regions are Ni nanoparticles and carbon matrices, respectively. (b) The scanning transmission electron microscope image showing the thin carbon coating on a Ni nanoparticle surface. (c) The CO2-to-CO conversion efficiencies at different applied potentials. Ni-NC_ATPA@C and Ni-NC_TPA@C are carbon-supported Ni nanoparticles derived from MOFs with 2-amino-terephthalic acid and terephthalic acid organic linkers, respectively. NC_ATPA@C is the Ni-free carbon powder derived from ATPA.
The CO2-to-CO conversion efficiencies of these Ni-C nanocomposites were the highest among all the reported carbon-supported Ni nanoparticles. The best Ni catalyst achieved a maximal efficiency of ~94% at an overpotential of 0.59 V, while previously reported Ni-C catalysts typically exhibited efficiencies lower than 25%. The significantly improved conversion efficiency was associated with the thin carbon coating. This coating prevented the Ni nanoparticles from directly contacting with aqueous electrolytes, and thus minimized hydrogen evolution reaction, a side reaction that decreased the conversion efficiency.
This work highlights the instrumental role of the surface carbon layers in promoting the CO2-reduction activity of Ni nanoparticles. The carbon-coating strategy could be extended to other low-cost transition metals, which may lead to a variety of cost-effective CO2-reduction catalysts.
To find out more please read:
Carbon-Supported Ni Nanoparticles for Efficient CO2 Electroreduction
Mingwen Jia, Changhyeok Choi, Tai-Sing Wu, Chen Ma, Peng Kang, Hengcong Tao, Qun Fan, Song Hong, Shizhen Liu, Yun-Liang Soo, Yousung Jung, Jieshan Qiu and Zhenyu Sun
Chem. Sci., 2018, 9, 8775-8780
Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.











