Scientists in Germany have reported a novel foldamer structure based on perylene bisimide (PBI) dyes and rigid oligophenylene ethynylene (OPE) backbones. The work was born out of the desire to create a well-defined macromolecular framework with predictable geometries. The group, from the University of Würzburg and lead by Frank Würthner, designed an OPE-fused PBI oligomer in which the π-π interactions between the molecules of PBI direct the folding geometry.
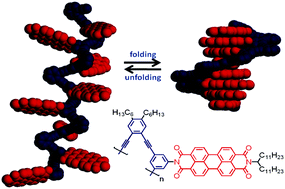
A typical OPE-fused PBI oligomer was found to contain between 8 and 9 PBI units, as determined by gel permeation chromatography and diffusion NMR. Further studies using MALDI-TOF ruled out impurities or side production in the oligomer. With this structural data in hand, they used UV-vis spectroscopy to prove that only the PBI, not the OPE, units participated in π-π stacking; a feature that is unique to their oligomer system. The authors then proved, through a series of elegant UV-vis analyses in chloroform and methylcyclohexane, that the oligomer was able to fold and unfold with changes in solvent polarity. These conclusions were also supported by molecular modeling studies.
The PBI oligomer reported in this work, in which the OPE backbone does not play a role in π-π stacking, resembles interactions in nucleic acids. Therefore, this system could be an ideal mimic for functional biological systems such as DNA or may also prove to have very interesting photophysical properties.
Find out more by downloading Würthner’s Edge article.
Posted on behalf of Patricia Pantos, Chemical Science web writer.