Huw Davies’ group have developed a domino sequence for the asymmetric synthesis of highly functionalised cyclopentanes.
The researchers from Emory University have combined scandium catalysis with a previously reported asymmetric rhodium-catalysed dienol formation to afford cyclopentanes in good to excellent levels of enantioselectivity. Four new stereogenic centres are formed in the reaction sequence and the cyclopentane products are obtained as single diastereoisomers.
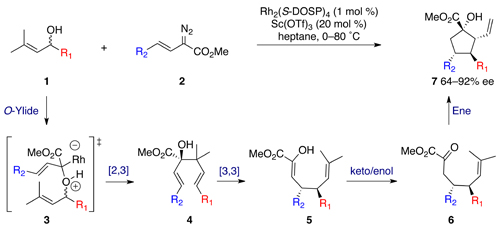
The one-pot procedure commences with a tandem oxygen ylide formation and [2,3] sigmatropic rearrangement between racemic allyl alcohol 1 and vinyl diazoacetate 2, resulting in formation of chiral dienol 4. Upon heating, an oxy-Cope rearrangement provides enol 5 in quantitative yield albeit with slight erosion of enantiomeric excess. Subsequent tautomerisation yields ketone 6, which is primed for a scandium triflate-catalysed carbonyl ene reaction on elevation of temperature, affording the cyclopentane product.
The impressive diastereoselectivity of this reaction combined with the complexity of the enantioenriched cyclopentane products highlights the synthetic power of reactive metal carbenoids in such thoughtfully designed reaction sequences. Download Davies’ Edge article to find out more.
“The discovery of new carbenoid reactions is always an exciting time: often the products generated are high energy and capable of further transformations. Needless to say, it was thrilling the first time the crude NMR indicated the domino sequence was viable.” Brendan Parr, from the Davies group
Posted on behalf of Alice E. Williamson, Chemical Science web writer.