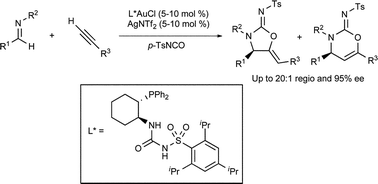
A racemic Au(I)-catalysed three-component reaction has been developed by US scientists to prepare cyclic carbamimidates from imines, terminal alkynes and sulfonylisocyanates. This reaction exploits the carbophilic π-acidity of gold catalysts to first activate an alkyne toward deprotonation and then to activate the internal alkyne generated toward intramolecular O-cyclisation.
Unlike similar previously reported multicomponent gold-catalysed reactions, the stereocentre generated during the alkynylation is preserved in the product. This trait was exploited by developing an enantioselective variant, using an unusual trans-1-diphenylphosphino-2-arylsulfamidocyclohexane ligand.
Moderate to excellent levels of enantioselectivity were obtained using a variety of N-arylbenzylidene anilines.
Find out more:
M J Campbell and F D Toste, Chem. Sci., 2011, DOI: 10.1039/c1sc00160d