Frontline therapies for treating colorectal cancer have shortcomings. These include their inability to impede local tumor recurrence and metastatic spread to distant sites such as the abdomen.
Researchers have now utilized a gene therapy approach that simultaneously compromises cancer cell survival while activating immune system cells with cancer-killing abilities.
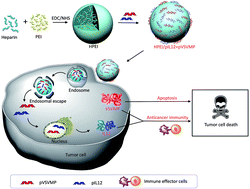
In the current study, the research team further armed with VSVMP gene delivery vessel with Interleukin-12 (IL-12) – a protein known to recruit and switch on the cancer-killing functions of immune cells.
The novel drug particles are based on Heparin-polyethyleneimine (HPEI) nanoparticles. To overcome the high toxicity and non-biocompatible nature of PEI, the team used a method to covalently conjugate this substance with heparin.
Their results, based on lab-grown cancer cells and animal studies, suggest that this novel complexed drug molecule (particle size: 53nm) increases tumor cell death, reduces division frequency, and stimulates the recruitment and activation of two types of cancer-killing cells: T cells and NK cells.
Specifically, the drug inhibited the growth of C-26 colon cancer cells. Animal studies showed that the drug reduced tumor weight. Metastatic spread of tumor cells to the abdomen was also reduced. The team proposes that the drug-derived IL-12 induces a secondary cascade of chemical mediators, which in turn recruit and activate cancer-killing immune cells. Their data supports this proposal. Interestingly, their study also found that the complexed drug molecule did not show adverse side effects within the major organs.
Read the full article here:
Nanoparticles co-delivering pVSVMP and pIL12 for synergistic gene therapy of colon cancer
Yuanyuan Xiao, Yuping Yang, Yujiao Wu, Chunmei Wang, Hao Cheng, Wei Zhao, Yang Li, Beibei Liu, Jianlin Long, Wenhao Guo, Guangping Gaoa and Maling Gou











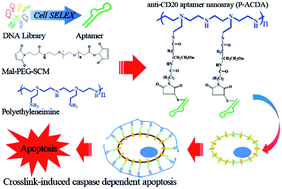
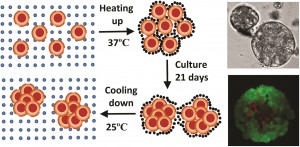
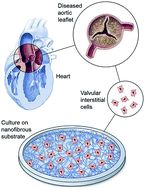 Within the body, VICs are arranged as monolayer sheets, forming a veneer on the outer surfaces of heart valve leaflets. They form this complex structure by depositing collagen – a cellular cementing protein that allows cells to interlock like a cobblestone path to form a sheet-like structure. The researchers reasoned that by artificially simulating conditions ideal for VIC growth within the body, they could create VIC sheets in the laboratory and examine their similarity to naturally occurring VICs in heart valve leaflets.
Within the body, VICs are arranged as monolayer sheets, forming a veneer on the outer surfaces of heart valve leaflets. They form this complex structure by depositing collagen – a cellular cementing protein that allows cells to interlock like a cobblestone path to form a sheet-like structure. The researchers reasoned that by artificially simulating conditions ideal for VIC growth within the body, they could create VIC sheets in the laboratory and examine their similarity to naturally occurring VICs in heart valve leaflets.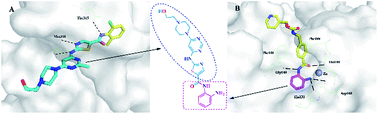 To determine the effect of the hybrid drugs on cancer cell survival, the research team tested the drug’s ability to halt the growth of three cell types exhibiting features of leukemia, kidney cancer and prostate cancer respectively. They found that all drugs in the series were toxic to cancer cells, with leukemia and kidney cancer cells showing the greatest degree of sensitivity to the hybrid drugs.
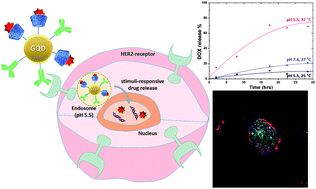
To determine the effect of the hybrid drugs on cancer cell survival, the research team tested the drug’s ability to halt the growth of three cell types exhibiting features of leukemia, kidney cancer and prostate cancer respectively. They found that all drugs in the series were toxic to cancer cells, with leukemia and kidney cancer cells showing the greatest degree of sensitivity to the hybrid drugs.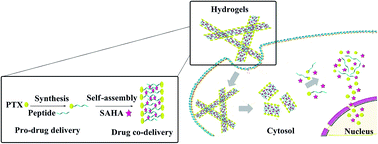 The researchers loaded PTX and SAHA onto the same nano-carrier in the following sequence: (1) an amino acid-based self assembling hydrogel precursor (Nap) was prepared, (2) PTX was conjugated to the self assembling hydrogel to form a pro-drug and (3) the pro-drug was allowed to encapsulate SAHA, forming the final drug (Nap-PTX-SAHA). The researchers subsequently characterized the mechanical features of their novel drug delivery system and tested it using a mouse model of liver cancer.
The researchers loaded PTX and SAHA onto the same nano-carrier in the following sequence: (1) an amino acid-based self assembling hydrogel precursor (Nap) was prepared, (2) PTX was conjugated to the self assembling hydrogel to form a pro-drug and (3) the pro-drug was allowed to encapsulate SAHA, forming the final drug (Nap-PTX-SAHA). The researchers subsequently characterized the mechanical features of their novel drug delivery system and tested it using a mouse model of liver cancer.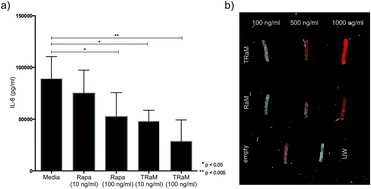 Their studies showed that the rapamycin-loaded nanoparticles were stable and biocompatible when tested in human endothelial cells. Further, the rapamycin release could be controlled by adjusting the pH values lower than 7 or higher than 8. The study found that the micelles were being taken up by cells within 6h after incubation. The study also demonstrates the specificity of the micelles by showing that what the cells were pre-treated with an integrin inhibitor, they were less likely to take up the micelles.
Their studies showed that the rapamycin-loaded nanoparticles were stable and biocompatible when tested in human endothelial cells. Further, the rapamycin release could be controlled by adjusting the pH values lower than 7 or higher than 8. The study found that the micelles were being taken up by cells within 6h after incubation. The study also demonstrates the specificity of the micelles by showing that what the cells were pre-treated with an integrin inhibitor, they were less likely to take up the micelles.