A researcher in the US has proposed a new way of computing bond order, which he says is more general and more consistently accurate across diverse kinds of materials.
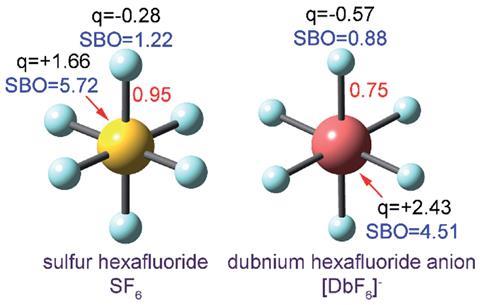
Source: © Royal Society of Chemistry SF6 contains six single-order S–F bonds and confirms that SF6transcends the Lewis octet rule that predicts four rather than six shared electron pairs around the central sulfur atom. The Db–F bond order (0.75) is lower than the S–F bond order (0.95)
Bond order quantifies how many electrons two atoms in a material share. But it’s a theoretical concept, not something you observe experimentally, so defining it and calculating it can get a bit fuzzy. ‘Some new chemistry students find it difficult to memorise which rules apply to which chemicals. For example, why should N2 and CF4 be described by a Lewis structure while triplet O2 and SF6are not?’ says Thomas Manz of New Mexico State University. ‘My method introduces a unifying principle where you no longer have to memorise different rules for different materials. All you have to do is calculate and you will get the accurate bond order,’ he explains.
Interested? The full story can be read in Chemistry World.
The original article can be read below and is free to access until the 13th November 2017:
Introducing DDEC6 atomic population analysis: part 3. Comprehensive method to compute bond orders
Thomas A. Manz
RSC Adv., 2017, 7, 45552-45581
DOI:10.1039/C7RA07400J










