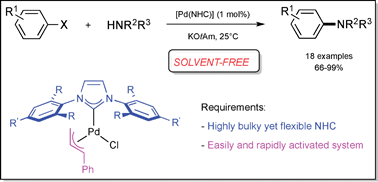
Steven P. Nolan’s group at the University of St Andrews in Scotland, UK, have reported a new solvent-free protocol for carrying out Buchwald-Hartwig aminations, an important class of reactions, for unactivated aryl chlorides using a palladium pre-catalyst. The reaction proceeds to complete conversion in around five minutes when initiated at room temperature, compared to zero conversion when using a solvent (DME). The secret to their success appears to be the use of a bulky yet flexible ligand, IPr*, in the pre-catalyst.
The reaction requires 1 mol% of the [Pd(NHC)] pre-catalyst – halving the amount of catalyst halved the conversion rate. An exotherm was observed in many cases: the reaction self-heated to 80ºC for a few seconds, therefore the group concludes that the protocol could be dangerous if carried out at a large scale. Interestingly the coupling could also be carried out with a solid substrate, leading to conversion of 73% after 24h, with no observed exotherm but a longer reaction time.
To learn more about this intriguing class of reactions, read the original article in RSC Advances:
Solvent-free aryl amination catalysed by [Pd(NHC)] Complexes, Anthony Chartoire, Arnaud Boreux, Anthony R. Martin and Steven P. Nolan, RSC Adv., 2013, 3, 3840–3843