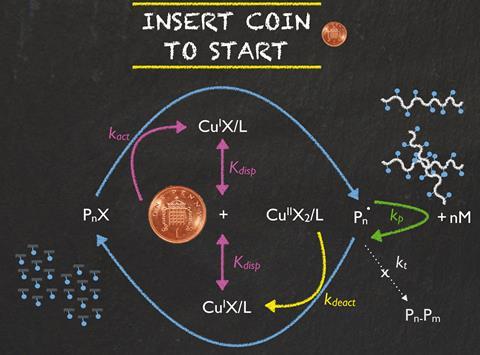
Scientists in the UK have discovered that a one-penny coin can catalyse polymerisations. The penny not only made the reaction start faster than the commonly used copper wire catalyst, but could also produce up to 50g of polymer in one batch.
Source: Royal Society of Chemistry
Single electron transfer living radical polymerisation is one of the most used reactions to produce everyday polymers such as polystyrene and acrylics. Polymerisation reactions need a catalyst to get them started – copper wire being the most common one. However, pure copper’s high cost can be restrictive.
To read the full article visit Chemistry World.
R. Aksakal, M. Resmini and C. R. Becer
Polym. Chem., 2016, Advance Article
DOI: 10.1039/C6PY01295G, Communication










