McKenzie et al. report the stability of a wide range of RAFT agents during photopolymerization.
The use of external stimuli to mediate the polymerization process has recently received significant attention with light being one of the most popular stimuli mainly due to its natural abundance and the possibility for spatiotemporal control. Photopolymerizations involving reversible addition fragmentation chain transfer (RAFT) have been widely investigated and studied exhibiting impressive characteristics such as fast reaction rates, good spatiotemporal control, and high-end group fidelity.
However, a report on the stability of these RAFT agents has been clearly missing from the literature. Qiao and co-workers recently discovered an initiator-free photopolymerization where the RAFT agent is activated by a blue LED. Following this work, they have investigated the photolytic stability of a range of RAFT agents under blue light irradiation. Careful NMR studies regarding the initiation process and the induction period revealed that the photopolymerization reaction is strongly dependent on the structure of the employed trithiocarbonates (TTCs).
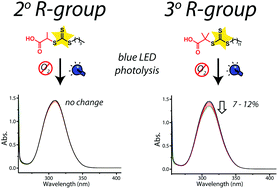
Degradation studies under polymerization relevant conditions showed that photolytic degradation of TTCs with more labile R-groups is observable within the reaction time scale up to 12% for a cyanosubstituted tertiary fragmenting group. On the contrary, when less stable (i.e. primary and secondary) R-group-derived radicals are employed, no degradation is detected.
Two main conclusions can be derived from these studies. Firstly, under identical photochemical conditions, the polymerization of acrylates will lead to higher end group fidelity polymers when compared to the polymerization of methacrylates. In addition, the induction period is dependent on the ability of the RAFT agent to fragment photolytically. As such, this work significantly contributes towards the understanding of the RAFT mechanism and side reactions during photopolymerization processes.
Tips/comments directly from the authors:
- The rate of photolysis, although demonstrated here under blue light irradiation (λmax ~ 460 nm) of constant intensity (ca. 1.5 mW/cm2), is likely strongly dependent on both the wavelength and intensity of the employed light source.
- Less stable (i.e. faster fragmenting) RAFT agents can be used with acrylate type monomers with minimal degradation due to conversion of the fragmenting species from tertiary to secondary during initiation.
- The rate of photopolymerization is also dependent on the initial RAFT agent concentration, and hence the targeted degree of polymerization.
- Trithiocarbonates are also more hydrolytically stable than many dithiobenzoates, so these photopolymerization reactions are also amenable to aqueous reaction conditions.
Read this exciting research for free until 31/08/2016 through a registered RSC account:
Investigation into the photolytic stability of RAFT agents and the implications for photopolymerization reactions
T. G. McKenzie, L. P. da M. Costa, Q. Fu, D. E. Dunstan and G. G. Qiao
Polym. Chem., 2016, 7, 4246-4253
DOI: 10.1039/C6PY00808A
—————-
Dr. Athina Anastasaki is a web writer for Polymer Chemistry. She is currently an Elings fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB).