Williams et al. utilise addition-fragmentation chain transfer aqueous dispersion polymerisation for the synthesis of bespoke cationic nano-objects directly in water.
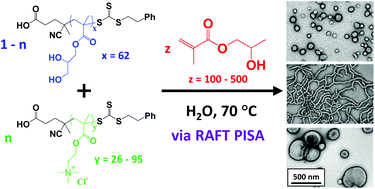
Polymerisation-induced self-assembly (PISA) via reversible addition-fragmentation chain transfer (RAFT) polymerisation enables the direct and efficient formation of various diblock copolymer morphologies (e.g. spherical micelles, work-like micelles, vesicles etc.) in aqueous solution. Here the first block is selected to be water-soluble, while the growing second block is water-insoluble and hence drives in situ self-assembly. This versatile approach can be conducted at much higher copolymer concentrations than traditional block copolymer self-assembly based on post-polymerisation processing.
Now Williams and co-workers report the synthesis of a range of cationic diblock copolymer nano-objects utilising a judicious binary mixture of chain transfer agents, namely non-ionic poly(glycerol monomethacrylate) (PGMA) and cationic poly[2-(methacryloyloxy)ethyl trimethylammonium chloride] (PQDMA) and using poly(2-hydroxypropyl methacrylate) (PHPMA) as the hydrophobic core-forming block. Systematic variation of the PQDMA mol fraction and the mean degree of polymerisation of the core-forming PHPMA block enabled the formation of well-defined spheres, worms or vesicles that remain cationic over a wide pH range.
Interestingly, higher cationic character led to the formation of kinetically-trapped spheres; this is because more effective electrosteric stabilisation prevents sphere-sphere fusion. In addition, using 5 mol% PQDMA stabiliser enabled preparation of a 12.5% w/w cationic worm gel that exhibited a zeta potential of +20 mV and a storage modulus of 137 Pa, as demonstrated by variable temperature rheology studies. This worm gel proved to be thermoresponsive: it underwent reversible degelation on cooling from 25 °C to 5 °C. Finally, such cationic gels exhibited weak antimicrobial activity towards the pathogen Staphylococcus aureus.
Tips/comments directly from the authors:
- It is really important to map out a detailed phase diagram for the reliable and reproducible identification of pure copolymer phases. This is particularly true for the elusive worm phase, since this occupies relatively narrow phase space.
- Using pairs of stabiliser blocks is a powerful and versatile means of tuning the copolymer morphology. If a wholly cationic stabiliser is used, only spheres can be obtained. However, using a binary mixture of a non-ionic and a cationic stabiliser allows access to cationic spheres, worms or vesicles. This is because the non-ionic stabiliser dilutes the charge density within the coronal layer. If maximum cationic character is desired, then the ionic block should have a higher degree of polymerisation than the non-ionic block. This will enable it to protrude from the layer of non-ionic stabiliser chains and influence the electrophoretic footprint of the diblock copolymer nano-objects.
- When diluting thermoresponsive worm dispersions to the relatively low concentrations typically used for TEM or DLS analysis, it is important for dispersions to be stored at ambient temperature. This is because the thermoresponsive degelation behaviour becomes irreversible below a certain critical copolymer concentration. Thus storing highly dilute (< 1 %) dispersions in a refrigerator at 4-5 °C simply leads to kinetically-trapped spheres – worms are no longer reformed on returning to ambient temperature under these conditions.
Read this exciting research for free until 31/07/2016 through a registered RSC account:
Bespoke cationic nano-objects via RAFT aqueous dispersion polymerisation
M. Williams, N. J. W. Penfold, J. R. Lovett, N. J. Warren, C. W. I. Douglas, N. Doroshenko, P. Verstraete, J. Smets and S. P. Armes
Polym. Chem., 2016, 7, 3864-3873
DOI: 10.1039/C6PY00696E
—————-
Dr. Athina Anastasaki is a web writer for Polymer Chemistry. She is currently an Elings fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB).