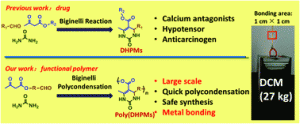
Zhao et al. describe the potential of the Biginelli polycondensation to improve metal bonding strength.
Recently, the introduction of efficient reactions (e.g. click reactions, Diels-Alder reactions) to polymer chemistry aiming to synthesize new condensation polymers with improved properties and characteristics has attracted considerable interest. However, on many occasions, the requirement of extensive synthetic steps (e.g. for monomer synthesis) in combination with the usage of unsafe reagents (e.g. toxic, explosive etc.) necessitates the need for the development of alternative strategies that will provide access to large scale functional materials. Towards this goal Tao and co-workers employed the Biginelli polycondensation reaction to polymerize a novel difunctional monomer consisting of benzaldehyde and beta-keto ester groups, to yield poly(dihydropyrimidin-2(1H)-one)s (poly(DHMPs) (Mn ~ 22000 g mol-1) within 1 h. Interestingly and in contrast with the small molecular DHMPs, the Biginelli polycondensates presented metal bonding capability and adhesive properties (up to ~ 2.8 Mpa). In addition, when monomers containing more functional groups were employed, stronger tensile shear strength was demonstrated indicating that the cross-linked polymer network has a positive effect on the bonding strength (3.9-5.9 Mpa). Finally, the efficiency of the reaction was further demonstrated by performing the reaction using an electric heat gun. The preparation of the monomers on a large scale, the facile nature of the polymerisation and the excellent metal bonding performance paves the way for the synthesis of new functional polymers.
Tips/comments directly from the authors:
1. When finishing the polycondensation of monomer AB, the final polymer should be precipitated into cold water immediately, because the viscosity of they system will increase after cooling down (solid can even be formed), which will make the purification challenging.
2. When precipitating the polymer, adding some base (e.g. NaOH) in water is helpful for the removal of the acetic acid. In addition, strong stirring during the precipitation is necessary to achieve satisfactory purification.
3. During the metal bonding test, evenly heating could improve the efficiency of bonding. An open system for the volatilization of water will also enhance the glue effect.
4. As the monomer A2B2 is viscous, gentle heating prior to use is helpful for measuring.
From drug to adhesive: a new application of poly(dihydropyrimidin-2(1H)-one)s via the Biginelli polycondensation, by Y. Zhao, Y. Yu, Y. Zhang, X. Wang, B. Yang, Y. Zhang, Q. Zhang, C. Fu, Y. Wei and L. Tao, Polym. Chem., 2015, 6, 4940-4945.
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Warwick (UK)/ Monash (Australia) research fellow working under the Monash Alliance. Visit http://haddleton.org/group-members for more information.