Truong et al. describe the synthesis of ultrahigh molecular weight and low polydispersity polystyrene diblock copolymers by RAFT-mediated emulsion polymerisation.
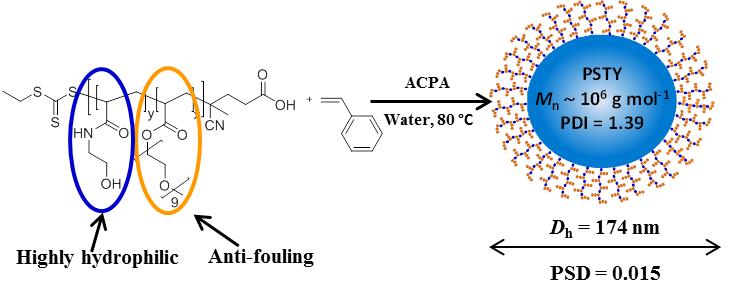
Ultra high molecular weight (UHMW) polymers have always been an ambitious target for synthetic polymer chemists. However, synthesising these materials using controlled radical polymerisation is challenging due to high levels of bimolecular termination and chain transfer to monomer that impede the growth of polymer chains. Monomers with higher propagation rate coefficient (kp) such as acrylamides and acrylates (and even methacrylates) have been successfully polymerised up to 106 g mol-1 but lower kp monomers (e.g. styrene) typically present broad molecular weight distributions when high molecular weight (e.g. 106 g mol-1) is targeted.
Truong et al. envisaged that a high polymerisation rate for the polymer chains would be required in order to produce well-defined UHMW polystyrene, whilst at the same time termination and side reactions would need to be minimised. The authors addressed this by employing the use of novel macromolecular chain transfer agents (CTA) in reversible addition fragmentation chain transfer polymerisation (RAFT)-mediated emulsion polymerisation. N-hydroxyethyl acrylamide (HEAA) and poly(ethylene glycol) methyl ether acrylate (PEGA) were copolymerised under judiciously selected reaction conditions to identify the most effective macrostabiliser for the emulsion polymerisation. The choice of these monomers proved crucial for the polymerisation, with PEGA conferring excellent antifouling characteristics while HEAA improves the water solubility of the macromolecular CTA and reduces partitioning of the macro-stabilisers within the styrene droplets and/or at the water/droplet interfaces. Under carefully optimised conditions, UHMW polystyrene of 106 g mol-1 could be obtained with relatively low dispersity values (<1.4) and unimodal molecular weight distributions even at near-quantitative conversions (>90%). Moreover, UV-Vis analysis confirmed the presence of the CTA, further suggesting that the reversible-deactivation radical polymerisation mechanism remained operative even to this very high conversion and molecular weight. Another interesting feature of this work is the linear relationship between particle size and molecular weight in this system, which seems to depart from the packing parameter theory, typically used to explain morphology transformations during emulsion or aqueous dispersion polymerisations. TEM analysis in all samples revealed uniformly spherical nanoparticles, even when the chain length of the polystyrene core was well above the threshold for the worm and vesicle formation. These experiments suggest that the packing parameter theory cannot be applied for all polymerisation-induced self-assembly systems and that further theoretical models are potentially required to fully understand the equilibrium morphology of soft nanoparticles.
In short, this article has overcome a longstanding challenge in the synthesis of UHMW polymers and created a new nanomaterial which offers great potential in numerous applications.
Summary points from the authors:
- 4,4′-Azobis(4-cyanopentanoic acid (ACPA) (free radical initiator) completely dissolves in water only after stirring for about 30 min. The stock ACPA solution should be made up fresh and not be stored for later use.
- To avoid the loss of styrene monomer (by evaporation) during polymerisation, there is no need to keep the emulsion polymerisation under the continuous flow of nitrogen.
- Samples prepared for dynamic light scattering measurements were not filtered, and as such filtration might enable a further reduction in the particle size distribution.
- The molar ratio of HEAA to PEGA in the macromolecular CTAs was optimised at 1 to 1. A higher molar ratio of HEAA to PEGA results in aggregation when targeting ultra-high molecular weight polystyrene. A lower molar ratio of HEAA to PEGA results in a higher portion of macromolecular CTAs partitioning within the styrene droplets.
Rapid synthesis of ultrahigh molecular weight and low polydispersity polystyrene diblock copolymers by RAFT-mediated emulsion polymerization by Nghia P. Truong, Marion V. Dussert, Michael R. Whittaker, John F. Quinn and Thomas P. Davis, Polym. Chem., 2015, 6, 3865-3874
Dr. Athina Anastasaki is a is a guest web-writer for Polymer Chemistry. She is currently a Warwick University (UK) and Monash University (Australia) research fellow working under the Monash Alliance. Visit the Haddleton group’s website for more information.










