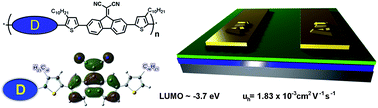
The past decade has witnessed extensive concentration on the synthesis of conjugated polymers and their applications for organic electronic devices due to their low-cost, light-weight, and flexibility compared to their inorganic semiconductor counterparts. Donor–acceptor (D–A) copolymerization is a currently common strategy to design and synthesize potential semiconducting copolymers. In this study, Yao and co-workers reported on the synthesis of four D–A copolymers on the basis of a novel acceptor (A) unit of 9-fluorenylidene malononitrile (CN2–Fluorene) and four common donor (D) units of 9-alkylfluorene (P1), benzodithiophene (P2), bithiophene (P3), and dithienopyrrole (P4). These four copolymers exhibit a weak ICT absorption edge up to 800 nm. The HOMO energy levels of the copolymers are finely tuned by the donor units, while the LUMO energy levels of the copolymers are highly depressed and determined by the CN2–Fluorene unit. The hole mobility of P3 is measured as 1.43 x 10-3 cm2.V-1.s-1 under ambient conditions and that of P2 and P4 is on the order of 10-4 cm2.V-1.s-1. The results reveal that CN2–Fluorene is a new electron-acceptor unit and may be incorporated with proper electron-donors when designing semiconducting D–A copolymers.
Synthesis and charge-transporting properties of electron-deficient CN2–fluorene based D–A copolymers by Jianhua Huang, Yan Zhao, Xunlei Ding, Hui Jia, Bo Jiang, Zhiguo Zhang, Chuanlang Zhan, Shenggui He, Qibing Pei, Yongfang Li, Yunqi Liu and Jiannian Yao Polym. Chem. 2012, 3, 2170-2177.
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistryon Twitter or Facebook.