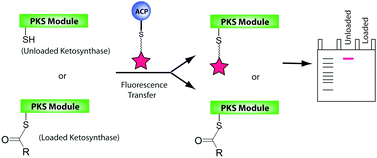
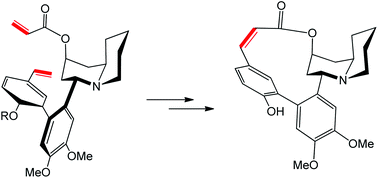
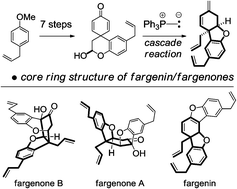
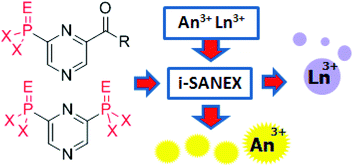
This month sees the following articles in Organic & Biomolecular Chemistry that are in the top ten most accessed:
PTSA-catalyzed Mannich-type–cyclization–oxidation tandem reactions: one-pot synthesis of 1,3,5-substituted pyrazoles from aldehydes, hydrazines and alkynes
Pei Liu, Ying-Ming Pan, Yan-Li Xu and Heng-Shan Wang
Org. Biomol. Chem., 2012, 10, 4696-4698
DOI: 10.1039/C2OB25487E
A new ratiometric and colorimetric chemosensor for cyanide anion based on Coumarin–hemicyanine hybrid
Zhenghao Yang, Zhipeng Liu, Yuncong Chen, Xiaoqing Wang, Weijiang He and Yi Lu
Org. Biomol. Chem., 2012, 10, 5073-5076
DOI: 10.1039/C2OB25516B
Highly enantioselective Biginelli reaction catalyzed by SPINOL-phosphoric acids
Fangxi Xu, Dan Huang, Xufeng Lin and Yanguang Wang
Org. Biomol. Chem., 2012, 10, 4467-4470
DOI: 10.1039/C2OB25663K
One-pot synthesis of 1,2,3-triazoles from boronic acids in water using Cu(II)–ß-cyclodextrin complex as a nanocatalyst
Babak Kaboudin, Yaghoub Abedi and Tsutomu Yokomatsu
Org. Biomol. Chem., 2012, 10, 4543-4548
DOI: 10.1039/C2OB25061F
The ligand and base-free Pd-catalyzed oxidative Heck reaction of arylboronic acids and olefins
Peng Sun, Yan Zhu, Hailong Yang, Hong Yan, Linhua Lu, Xiang Zhang and Jincheng Mao
Org. Biomol. Chem., 2012, 10, 4512-4515
DOI: 10.1039/C2OB25462J
Very high stereoselectivity in organocatalyzed desymmetrizing aldol reactions of 3-substituted cyclobutanones
David J. Aitken, Angela M. Bernard, Francesca Capitta, Angelo Frongia, Régis Guillot, Jean Ollivier, Pier Paolo Piras, Francesco Secci and Marco Spiga
Org. Biomol. Chem., 2012, 10, 5045-5048
DOI: 10.1039/C2OB25813G
First total synthesis of (+)-indicanone
Yujiro Hayashi, Kumiko Ogawa, Fuyuhiko Inagaki and Chisato Mukai
Org. Biomol. Chem., 2012, 10, 4747-4751
DOI: 10.1039/C2OB25500F
Strategy for catch and release of azide-tagged biomolecules utilizing a photolabile strained alkyne construct
Martin Golkowski, Carlo Pergola, Oliver Werz and Thomas Ziegler
Org. Biomol. Chem., 2012, 10, 4496-4499
DOI: 10.1039/C2OB25440A
Synthesis of diversely 1,3,5-trisubstituted pyrazoles via 5-exo-dig cyclization
Dmitry A. Borkin, Mirela Puscau, Alena Carlson, Agnes Solan, Kraig A. Wheeler, Béla Török and Roman Dembinski
Org. Biomol. Chem., 2012, 10, 4505-4508
DOI: 10.1039/C2OB25580D
Thiol–yne coupling: revisiting old concepts as a breakthrough for up-to-date applications
Alessandro Massi and Daniele Nanni
Org. Biomol. Chem., 2012, 10, 3791-3807
DOI: 10.1039/C2OB25217A
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Organic & Biomolecular Chemistry? Then why not submit to us today or alternatively email us your suggestions.











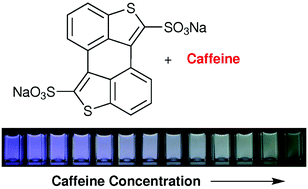 Scientists in Switzerland have synthesised a dye (3,4:3’,4’-bibenzo[b]thiophene-2,2’-disulfonate) that can be used as a sensitive and selective molecular probe for the fluorimetric detection of caffeine in water.
Scientists in Switzerland have synthesised a dye (3,4:3’,4’-bibenzo[b]thiophene-2,2’-disulfonate) that can be used as a sensitive and selective molecular probe for the fluorimetric detection of caffeine in water.