It comes as no surprise to those with a background in organic or medicinal chemistry that one of the most important and often-overlooked synthetic transformations is the formation of amide bonds.
Amide linkages are one of the most prolific moieties in the synthesis of pharmaceuticals and biologically active molecules. However, despite their prevalence there remain synthetic challenges, as even the simplest amides can be difficult to make.
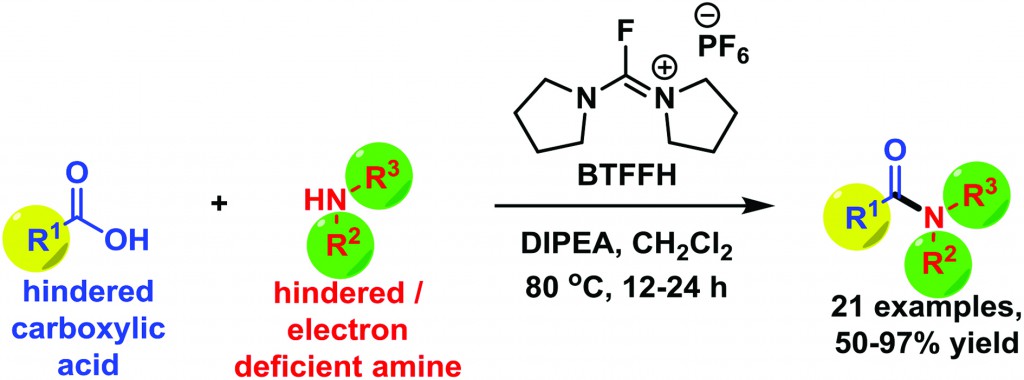
A group at the University of Southern Denmark led by Prof. Trond Ulven has developed a protocol for amide coupling through in situ formation of acyl fluorides.
 |
Initially, the researchers were working toward the synthesis of a molecular inhibitor for the free fatty acid receptor 2 (FFA2/GPR43), which has recently generated some interest as a target for treating various metabolic disorders.
While attempting the synthesis of an intermediate, coupling between their sterically hindered and sensitive carboxylic acid with an electron deficient and hindered amide understandably led to unsatisfactory results using standard coupling procedures.
Given the multiple steps required to generate both intermediates, the group decided to explore alternative methods to solve their problem. Indeed, acyl fluorides proved to be ideal as they behave like activated esters due to the unique nature of the carbonyl-fluoride bond while also minimizing steric hindrance between the two coupling partners.
Literature protocols are available for the generation of acyl fluorides and there are disadvantages associated with some. In recent years however, a number of alternative fluorinating agents have been reported that are capable of generating the acyl fluoride in situ under mild reaction conditions.
Prof. Ulven’s group was able to further improve the efficiency of this methodology by utilizing an alternative fluorinating agent, BTFFH, which is normally used in solid-phase peptide synthesis. This reagent reduces byproduct formation observed with reagents such as DAST, Deoxo-Fluor and XtalFluor-E. High conversions and isolated yields were obtained as a result and Ulven’s method was also successfully applied to amide coupling reactions previously reported as low yielding.
There is still a need for chemists to develop better ways to synthesize complex amide-containing structures without the need for external reagents. In the meantime, solutions such as these overcome synthetic challenges and are critical to further development and understanding in organic reaction design.
To find out more see:
A protocol for amide bond formation with electron deficient amines and sterically hindered substrates
Maria E. Due-Hansen, Sunil K. Pandey, Elisabeth Christiansen, Rikke Andersen, Steffen V. F. Hansen and Trond Ulven
DOI: 10.1039/C5OB02129D
Victoria Corless is currently completing her Ph.D. in organic chemistry with Prof. Andrei Yudin at The University of Toronto. Her research is centred on the synthesis of kinetically amphoteric molecules, which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules.










