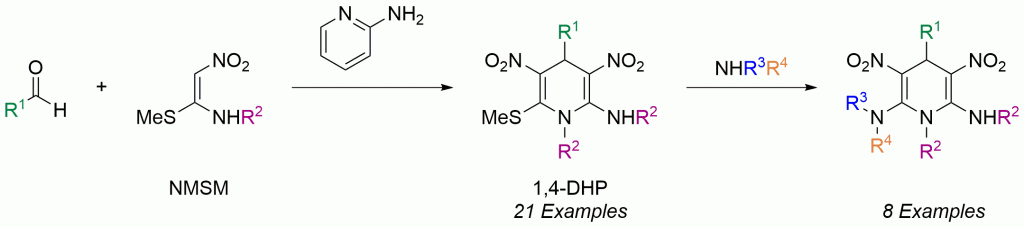
Not many molecules can claim to be both a Michael donor and Michael acceptor while containing a good leaving group as is the case with nitroketene-N,S-acetals like N-methyl-S-methyl nitroethylene (NMSM), broadly categorized as nitroenamines.
As a double actor in the pseudo three-component reaction investigated by Rao and Parthiban, moieties of NMSM react three times in two roles: twice as a nucleophile and once as an electrophile. The other components in the same pot included the aromatic aldehyde and catalytic 2-aminopyridine.
The methodology generates an alternate route from the classic Hantzsch reaction to the 1,4-dihydropyridine (DHP) platform with a broader scope and purification by recrystallization. Advantageously, 1,4-DHPs are calcium channel blockers and hydride sources for reduction.
The functionally-rich NMSM component conveniently diversifies DHP which could lead to more potent medicinal applications or pesticides.
The two inherent diversity points on the platform arose from the aldehyde and amine precursors. Additional diversity from amine substitution of the built-in sulfide leaving group created a total of 29 unique 1,4-DHP possibilities.
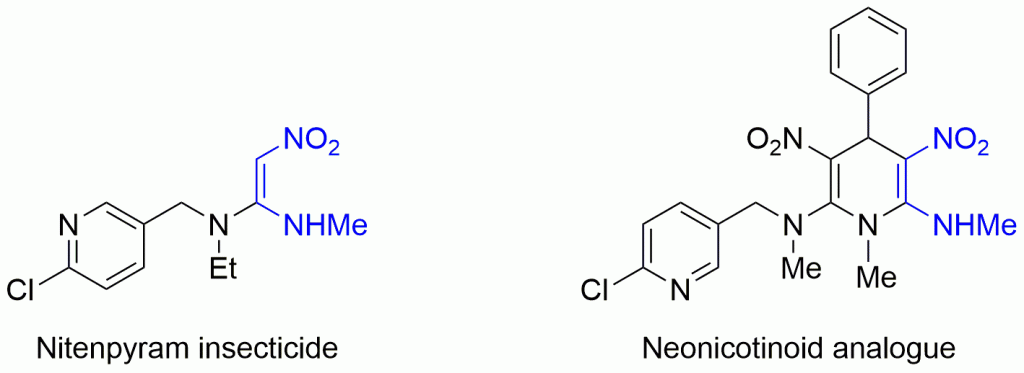
Specifically, they synthesized a neonictinoid insecticide analogue to the popular nitenpyram compound to constrain the alkene to the cis-isomer with muted toxicity to mammals.
Overall, leveraging an electronically versatile synthon like NMSM in a multicomponent reaction leads to enhanced diversity for analoging with myriad compounds that could perform better than those currently available.
To find out more see:
One-pot pseudo three-component reaction of nitroketene-N,S-acetals and aldehydes for synthesis of highly functionalized hexa-substituted 1,4-dihydropyridines
H. Surya Prakash Rao and A. Parthiban
DOI: 10.1039/c4ob00628c
 Jennifer Lee is currently a Ph.D. candidate in Dr. George Kraus’ organic chemistry lab at Iowa State University. Her research focuses on designing methodologies to transform carbohydrate-derived biomass into biorenewable commodity and specialty chemicals. The creation of a versatile platform technology led to diverse and industrially-relevant aromatic compounds to work toward a more sustainable future.
Jennifer Lee is currently a Ph.D. candidate in Dr. George Kraus’ organic chemistry lab at Iowa State University. Her research focuses on designing methodologies to transform carbohydrate-derived biomass into biorenewable commodity and specialty chemicals. The creation of a versatile platform technology led to diverse and industrially-relevant aromatic compounds to work toward a more sustainable future.