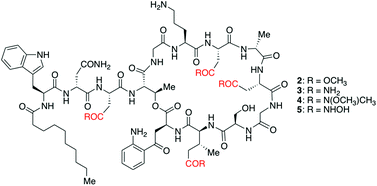
The emergence of multi-drug resistant bacterial infections has created a pressing need for the identification of new drugs. This HOT article describes the chemical modification of daptomycin, an antibiotic used to treat Gram-positive bacterial infections. Because of its anionic character daptomycin has a high affinity to pulmonary surfactants, but this limits its use in the treatment of pulmonary infections.
Scott Miller and co-workers hypothesized that reducing surfactant interactions may increase the antibiotic activity of daptomycin. Consequently, they set out to convert the daptomycin carboxylic acid moieties to carboxylate isosteres. This paper reports a direct and efficient procedure to produce isostere analogues of daptomycin, suppressing backbone-cyclization side reactions. The use of a high resolution UPLC-MS/MS technique to characterise the synthetic products by fragmentation analysis is also described.
An efficient chemical synthesis of carboxylate-isostere analogs of daptomycin
Sabesan Yoganathan, Ning Yin, Yong He, Michael F. Mesleh, Yu Gui Gu and Scott J. Miller
DOI: 10.1039/C3OB40924D
Free to access for 4 weeks