In this HOT OBC Communication Mark E. Graham, Richard J. Payne and co-workers probe the effect of a recently discovered post-translational modification of threinine of the assembly protein AP180, a protein which plays a crucial role in clathrin coated vesicle formation in synaptic vesicle endocytosis.
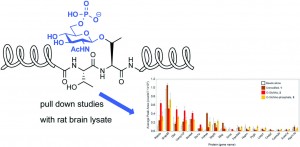
Using pull down experiments on AP180 peptide fragments they aimed to explore how binding to proteins in rat brain lysate is influenced by this modification.
Read the article to find out more about the peptides used and the effect of this modification on the affinity towards synaptic proteins.
Go on, it’s FREE to access for the next 4 weeks!
Synthesis and protein binding studies of a peptide fragment of clathrin assembly protein AP180 bearing an O-linked β-N-acetylglucosaminyl-6-phosphate modification
Mark E. Graham, Robin S. Stone, Phillip J. Robinson and Richard J. Payne
Org. Biomol. Chem., 2012, 10, 2545-2551
DOI: 10.1039/C2OB07139H










